A kind of organozinc complex catalyst and its preparation method and application
A zinc complex and complex technology, applied in the direction of zinc organic compounds, etc., can solve the problems of no isotactic selectivity and low activity, and achieve the effects of simple and easy preparation method, high isotactic selectivity and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
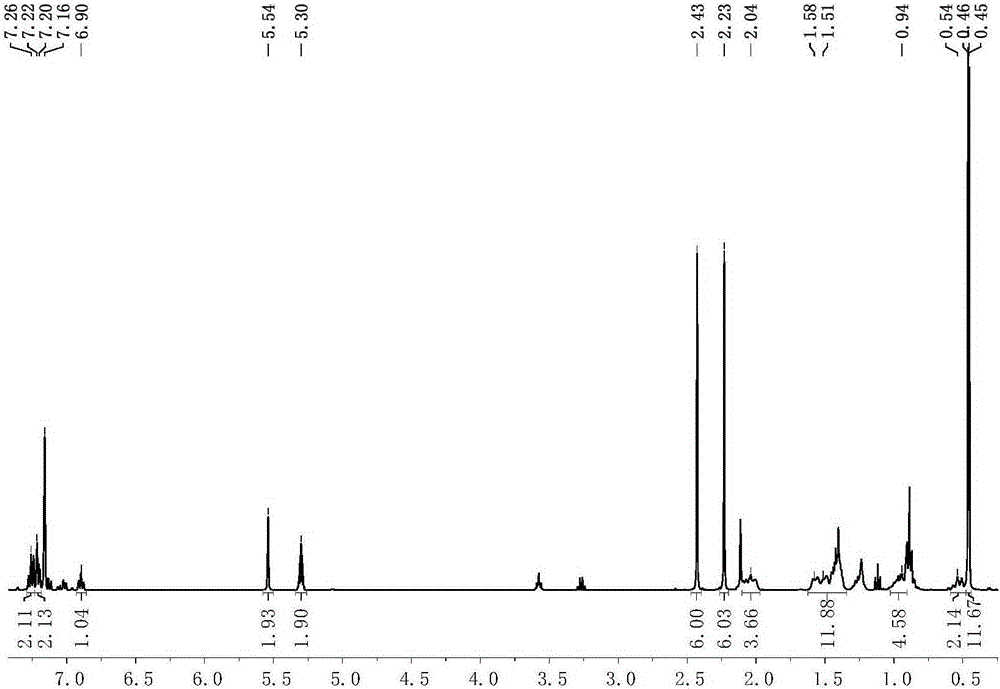
[0077] Preparation of scorpion-type zinc complexes 1-2
[0078] The process of preparing the scorpion-type zinc complexes 1-2 in this embodiment is shown in the general reaction formula (Ⅲ):
[0079]
[0080] Under anhydrous and oxygen-free conditions, dissolve 0.001 mole of the scorpion-type ligand with the structure of formula (II) in tetrahydrofuran, cool down to -30°C, and add n-butyllithium with a concentration of 1.0mol / L to it. 1.0 mL of n-hexane solution was reacted for 30 minutes, the resulting mixed liquid was added dropwise in the tetrahydrofuran suspension of zinc dichloride, reacted at room temperature for 3 hours, and then potassium amide [KN(SiXMe 2 ) 2 ], after 5 hours of reaction, the solvent was removed under reduced pressure, 20 mL of toluene was added thereto for extraction, filtered, the filtrate was concentrated to 2 mL, 1 mL of n-hexane was added, and recrystallized in a refrigerator at -30 ° C. After 24 hours, a large amount of white solid was preci...
Embodiment 2
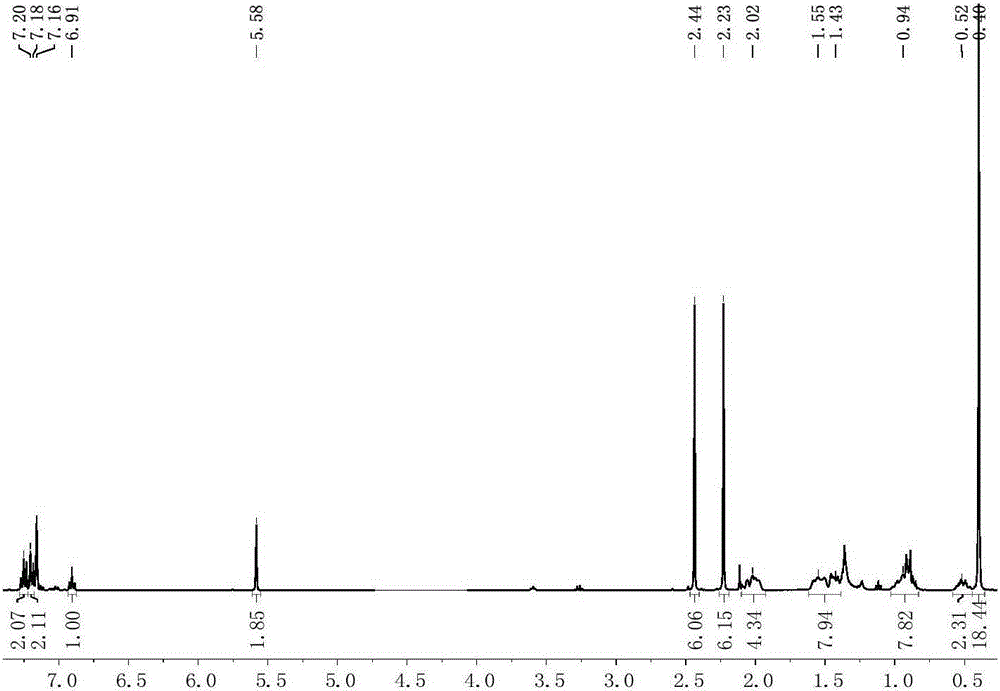
[0085] Preparation of scorpion-type zinc complexes 3-6
[0086] The process of preparing the scorpion-type zinc complexes 3-6 in this embodiment is shown in the general reaction formula (IV):
[0087]
[0088] Under anhydrous and oxygen-free conditions, dissolve 0.001 mole of the scorpion-type ligand with the structure of formula (II) in tetrahydrofuran, and add 0.001 mole of NaH solid to react for 30 minutes, and add the obtained mixed solution dropwise to dichloride In the tetrahydrofuran suspension of zinc, react at 50°C for 5 hours, then add alkoxy potassium salt dropwise to it, react for 2 hours, remove the solvent under reduced pressure, add 20mL of toluene to it for extraction, filter, concentrate the filtrate to 2mL, add 1mL n-Hexane, put it in a -30°C refrigerator for recrystallization, and a large amount of white solid precipitated after 24 hours. Finally, the solid was separated, washed twice with n-hexane, and vacuum-dried for 2 hours to obtain a scorpion-type ...
Embodiment 3
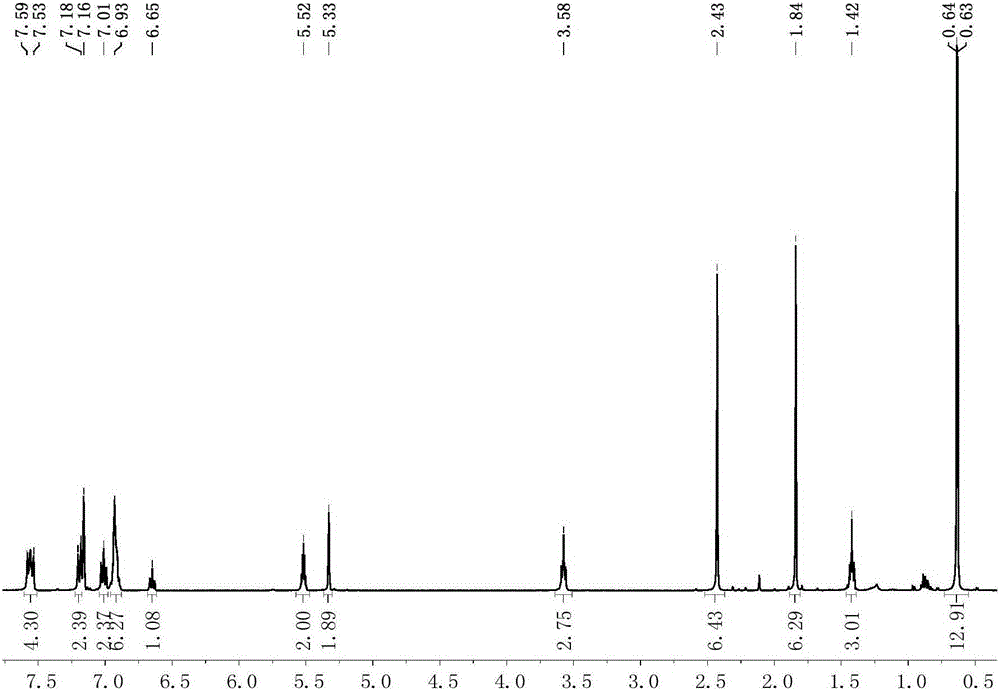
[0094] Preparation of scorpion-type zinc complexes 7-8
[0095] The process of preparing the miscellaneous scorpion-type zinc complexes 7-8 in this embodiment is shown in the general reaction formula (Ⅴ):
[0096]
[0097] Under anhydrous and oxygen-free conditions, dissolve 0.001 mole of the scorpion-type ligand with the structure of formula (II) in tetrahydrofuran, cool down to -30°C, and add n-butyllithium with a concentration of 1.0mol / L to it. 1.0 mL of n-hexane solution was reacted for 30 minutes, and the resulting mixed liquid was added dropwise in the tetrahydrofuran suspension of zinc dichloride, and reacted at room temperature for 5 hours, and then potassium amide [KN(SiXMe 2 ) 2 ], after 2 hours of reaction, the solvent was removed under reduced pressure, 20 mL of toluene was added thereto for extraction, filtered, the filtrate was concentrated to 2 mL, 1 mL of n-hexane was added, and recrystallized in a refrigerator at -30 ° C. After 24 hours, a large amount of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com