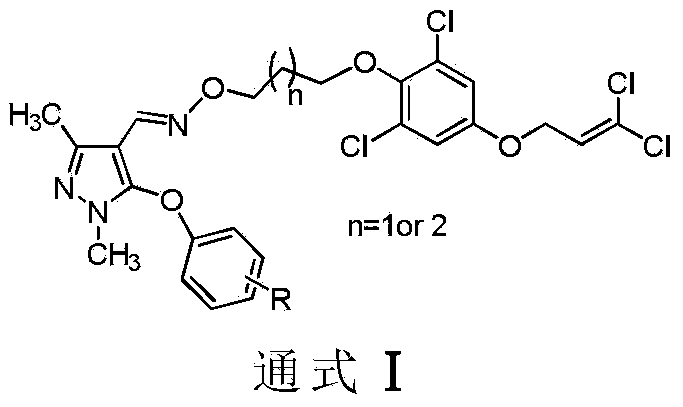
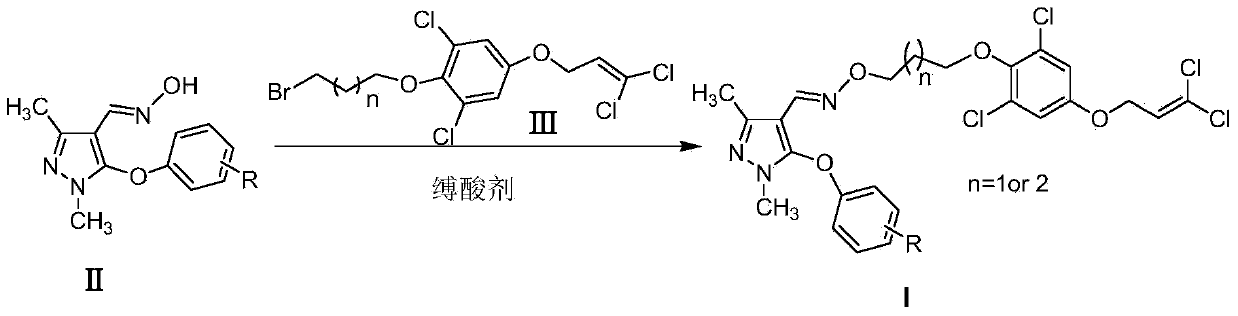
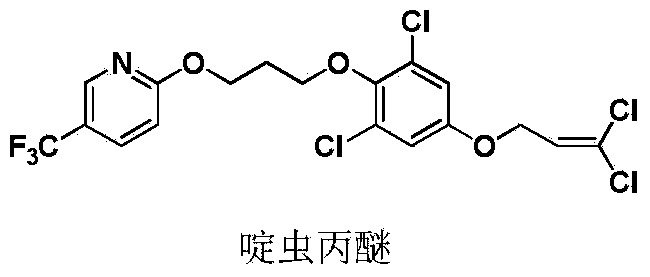
Pyrazole oxime derivative containing dichloropropene as well as preparation method and application method of derivative
A technology of dichloropropene and pyrazole oxime is applied in the field of pesticides to achieve excellent control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of Compound Ia (n=1, R=3-F)
[0034]
[0035] 5 mmol of compound II was dissolved in an appropriate amount of N,N-dimethylacetamide, and 6 mmol of compound III and 10 mmol of cesium carbonate were added thereto. Heat to reflux for 18h, and TLC detects that the reaction is complete. After filtration, the filtrate was concentrated under reduced pressure. The crude product was dissolved in dichloromethane, washed with water, dried, concentrated, and separated by column chromatography to obtain the target product Ia as a light yellow oil. 1 H NMR (400MHz, CDCl 3 )7.78(s,1H,CH=N),7.23-7.29(m,1H,ArH),6.78-6.82(m,3H,ArH),6.63-6.69(m,2H,ArH),6.11(t,J =6.4Hz,1H, CH CH 2 ), 4.57 (d, J=6.4Hz, 2H, CHC H 2 O), 4.22(t, J=6.4Hz, 2H, CH 2 OAr), 3.99(t, J=6.4Hz, 2H, C H 2 O-N=CH),3.62(s,3H,N-CH 3 ),2.38(s,3H,CH 3 ),2.04-2.11(m,2H,CH 2 C H 2 CH 2 ).
Embodiment 2
[0037] Synthesis of compound Ib (n=1, R=4-F)
[0038]
[0039] Dissolve 5mmol of compound II in an appropriate amount of acetonitrile, add 6.5mmol of compound III and 15mmol of K 2 CO 3 . Heat to reflux for 15h, and TLC detects that the reaction is complete. After filtration, the filtrate was concentrated under reduced pressure. The crude product was dissolved in dichloromethane, washed with water, dried, concentrated, and separated by column chromatography to obtain the target product Ib as a light yellow oil. 1 H NMR (400MHz, CDCl 3 )7.78(s,1H,CH=N),6.86-7.04(m,4H,ArH),6.84(s,2H,Ar-H),6.13(t,J=6.0Hz,1H,C H CH 2 ), 4.60 (d, J=4.0Hz, 2H, CHC H 2 O), 4.24(t, J=6.0Hz, 2H, C H 2 OAr), 4.01(t, J=6.0Hz, 2H, C H 2 O-N=CH),3.63(s,3H,N-CH 3 ),2.39(s,3H,CH 3 ),2.06-2.13(m,2H,CH 2 C H 2 CH 2 ).
Embodiment 3
[0041] Synthesis of compound Ic (n=1, R=4-OCF 3 )
[0042]
[0043] 5 mmol of compound II was dissolved in an appropriate amount of N,N-dimethylformamide, and 7 mmol of compound III and 14 mmol of 4-N,N-lutidine were added thereto. Heat to reflux for 20h, and TLC detects that the reaction is complete. After filtration, the filtrate was concentrated under reduced pressure. The crude product was dissolved in dichloromethane, washed with water, dried, concentrated, and separated by column chromatography to obtain the target product Ic as a light yellow oil. 1 H NMR (400MHz, CDCl 3)7.70(s,1H,CH=N),7.09(d,J=8.8Hz,2H,ArH),6.84(J=9.2Hz,2H,ArH),6.75(s,2H,Ar-H),6.03 (t,J=8.0Hz,1H,C H CH 2 ), 4.50 (d, J=6.4Hz, 2H, CHC H 2 O), 4.12(t, J=6.4Hz, 2H, C H 2 OAr), 3.91(t, J=6.4Hz, 2H, C H 2 O-N=CH),3.55(s,3H,N-CH 3 ),2.30(s,3H,CH 3 ),1.95-2.00(m,2H,CH 2 C H 2 CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com