Modulators of the g protein-coupled mas receptor and the treatment of disorders related thereto
A solvate, selected technology, used in sexual diseases, anti-inflammatory agents, metabolic diseases, etc., can solve problems such as weak binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0355] Some Embodiments: Compositions and Methods Related to Compounds of the Invention
[0356] One aspect of the invention pertains to compositions comprising a compound of the invention. One aspect of the present invention pertains to a pharmaceutical product selected from the group consisting of pharmaceutical compositions, formulations, unit dosage forms and kits, each comprising a compound of the present invention. One aspect of the present invention pertains to pharmaceutical compositions comprising a compound of the present invention and a pharmaceutically acceptable carrier. One aspect of the present invention pertains to a process for the preparation of a pharmaceutical composition comprising the step of admixing a compound of the present invention and a pharmaceutically acceptable carrier; some embodiments pertain to a pharmaceutical composition obtainable by any of the methods described herein. One aspect of the invention pertains to compositions comprising a comp...
Embodiment 1
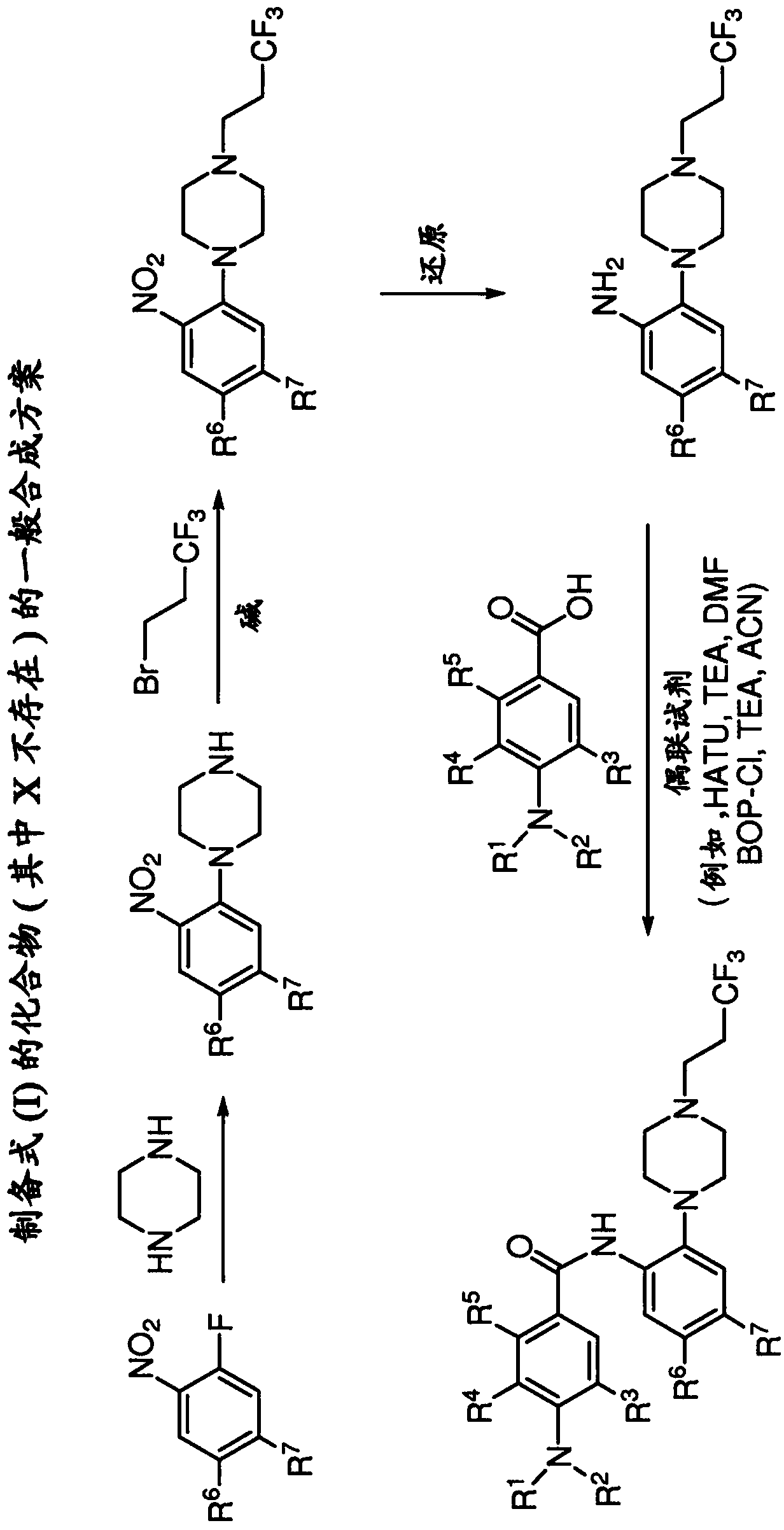
[0625] Embodiment 1: the synthesis of compound of the present invention
[0626] Exemplary syntheses for compounds of the invention are shown in Figures 1 to 4C , where the variable R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 and "X" have the same definition used throughout this disclosure.
[0627] The compounds of the present invention and their synthesis are further illustrated by the following examples. The following examples are provided to further define the invention without limiting the invention to the specifics of these examples. The compounds described in the context of this application were named according to AutoNom Version 2.2, AutoNom 2000, CS ChemDraw Ultra Version 7.0.1 or CS ChemDraw Ultra Version 9.0.7. In some instances, common names are used and it is understood that such common names will be known to those skilled in the art.
[0628] Proton NMR ( 1 H NMR) spectra were recorded with a Bruker Avance-400 equipped with QNP (Quadruple Probe) or BBI (B...
Embodiment 11
[0630] Example 1.1: Preparation of N-(4-chloro-2-(4-(3,3,3-trifluoropropyl)piperazin-1-yl)phenyl)-4-((diethylamino)methyl base) benzamide (compound 2).
[0631] Step A: Preparation of 4-chloro-2-(4-(3,3,3-trifluoropropyl)piperazin-1-yl)aniline.
[0632] Piperazine (36.8 g, 427 mmol) was dissolved in IPA (150 mL) and cooled in an ice bath. 4-Chloro-2-fluoro-1-nitrobenzene (25 g, 142 mmol) previously dissolved in IPA (100 mL) was slowly added to the reaction mixture via a drop funnel (the solution turned yellow-orange over time). After the addition was complete, the reaction mixture was warmed to room temperature and stirred at this temperature overnight. The next day, the solvent was evaporated and the product extracted (H 2 200 mL each of O and EtOAc). The aqueous layer was extracted twice with EtOAc (200 mL). The organic layers were combined and washed with H 2 O / brine (500 mL) back extracted once. The organic layer was washed with MgSO 4 Drying and concentration gave...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com