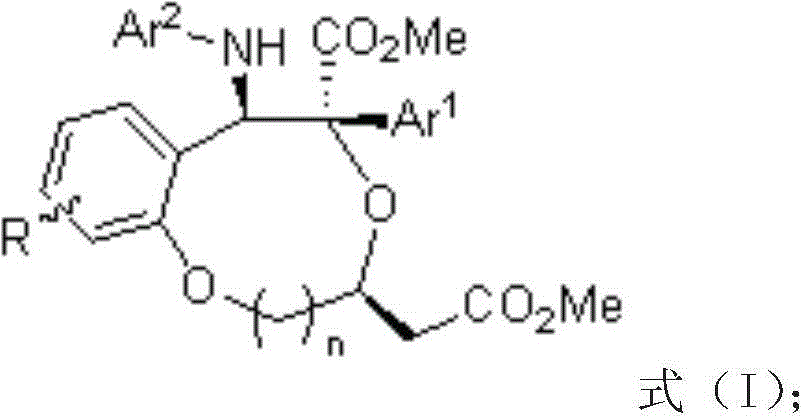
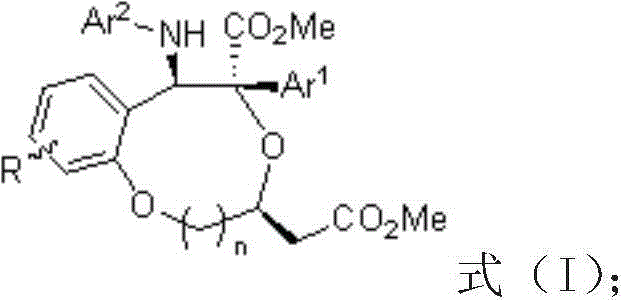
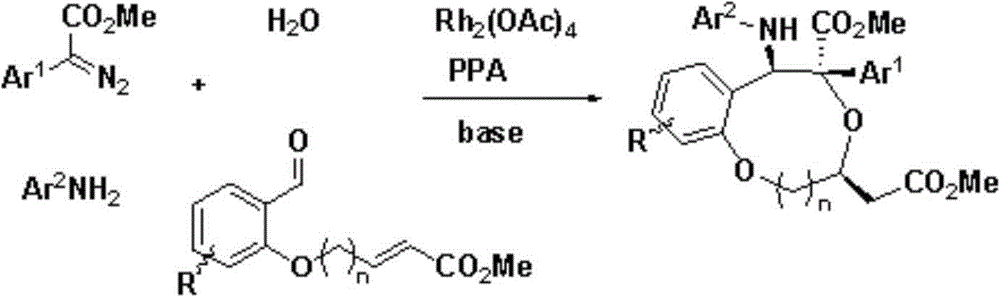
Benzo dioxy heterocyclic derivatives with optical activity and preparation method and application thereof
A benzodioxane and optically active technology, which is applied in the field of preparation of benzodioxane derivatives, can solve the problems of cumbersome synthesis methods and large operation risks, and achieve high yield, high selectivity, Compositing cheap effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Aromatic aldehyde 1:
[0042] Weigh aromatic aldehyde 1 (0.20mmol), rhodium acetate (1.00mg, 0.002mmol), chiral phosphoric acid A (8.6mg, 0.01mmol) p-bromoaniline (0.30mmol), Molecular sieves (100 mg) put them into a small test tube reactor, add redistilled 2.0 ml of dichloromethane, and stir at room temperature for 2 hours. Weigh phenyldiazoacetate methyl ester (0.30mmol) and dissolve it in 1.0ml redistilled methylene chloride, and inject it into the reaction system through a peristaltic pump for 1 hour, continue to stir for 2 hours after the injection, filter, and add 1 , 8-diazabicyclo[5.4.0]undec-7-ene (0.2mmol), stirred overnight at room temperature, and then passed column chromatography (eluent: petroleum ether: ethyl acetate=1:20~ 1:5) were isolated to obtain optically active benzodioxane derivatives
[0043]
[0044] 3.49(s, 3H), 3.10(qd, J=15.3, 4.4Hz, 2H).
[0045] 13 C NMR (101MHz, CDCl 3 )δ168.79,168.26,156.22,144.30,136.60,130.87,130.62,129.67,12...
Embodiment 2
[0047] Aromatic aldehyde 1:
[0048] Weigh aromatic aldehyde 1 (0.20mmol), rhodium acetate (1.00mg, 0.002mmol), chiral phosphoric acid B (5.0mg, 0.01mmol) p-bromoaniline (0.30mmol), Molecular sieves (100 mg) put them into a small test tube reactor, add redistilled 2.0 ml of dichloromethane, and stir at room temperature for 2 hours. Weigh phenyldiazoacetate methyl ester (0.30mmol) and dissolve it in 1.0ml redistilled methylene chloride, and inject it into the reaction system through a peristaltic pump for 1 hour, continue to stir for 2 hours after the injection, filter, and add 1 , 8-diazabicyclo[5.4.0]undec-7-ene (0.2mmol), stirred overnight at room temperature, and then passed column chromatography (eluent: petroleum ether: ethyl acetate=1:20~ 1:5) The optically active benzodioxane derivative 2A was isolated. The yield was 48%, the dr value was greater than 95:5, and the ee was 78%.
[0049]
Embodiment 3
[0051] Weigh aromatic aldehyde 1 (0.20mmol), rhodium acetate (0.002mmol), p-bromoaniline (0.30mmol), chiral phosphoric acid A (0.01mmol), Molecular sieves (100 mg) put them into a small test tube reactor, add redistilled 2.0 ml of dichloromethane, and stir at room temperature for 2 hours. Take by weighing p-methylphenyldiazoacetic acid methyl ester (0.30mmol) and dissolve in 1.0ml redistilled dichloromethane, and inject in the reaction system by peristaltic pump 1 hour, after injecting, continue to stir for 2 hours, filter, to Add 1,8-diazabicyclo[5.4.0]undec-7-ene (0.2mmol) to the filtrate, stir overnight at room temperature, then pass through column chromatography (eluent: petroleum ether: ethyl acetate=1 :20~1:5) The optically active benzodioxane derivative pure product 2B was isolated. The yield was 46%, the dr value was greater than 95:5, and the ee was 90%.
[0052]
[0053] 15.4, 5.5Hz, 2H).
[0054] 13 C NMR (101MHz, CDCl 3 )δ168.84,168.27,156.25,143.92,136.67...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com