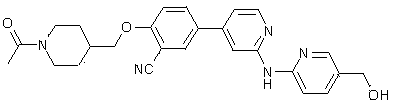
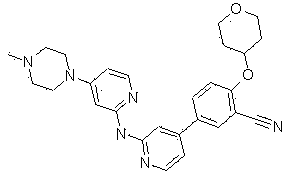
Benzonitrile derivatives as kinase inhibitors
A technology of compounds, mixtures, applied in techniques and procedures, capable of solving problems such as increased kinase expression, high protein kinase activity, increased kinase activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 105-117
[0766] Analytical method:
[0767] LCMS analysis:
[0768] Method A: A-0.1% TFA in H 2 O, B - 0.1% TFA in CAN: flow rate 2.0 ml / min.
[0769] Column: XBridge C8 (50 x 4.6mm, 3.5 μ)
[0770] Method B: A-10mM NH 4 HCO 3 , B: ACN; flow rate: 1.0 ml / min
[0771] Column: XBridge C8 (50x4.6mm, 3.5μ),
[0772] 1 H NMR:
[0773] Bruker 400 MHz
[0774] HPLC:
[0775] Method A:
[0776] Method: A-0.1% TFA in H 2 O, B-0.1% TFA in ACN: Flow rate – 2.0 ml / min.
[0777] Column: XBridge C8 (50 x 4.6 mm, 3.5 μ).
[0778] Synthesis of 5-bromo-2-cyclopropylmethoxybenzonitrile
[0779]
[0780] Sodium hydride, 60% suspended in oil (3.6 g, 0.09 mol) was added to a solution of cyclopropylmethanol (6.49 g, 0.09 mol) in dry DMF (200 ml) at 0 °C under nitrogen middle. After 30 min, 5-bromo-2-fluorobenzonitrile (12.0 g, 0.06 mol) in dry DMF (50 ml) was added at 0 °C and the reaction was stirred at 50 °C for 16 h. Ice water (200 ml) was added to the reaction mixture and extracted w...
Embodiment A
[0939] Example A: Injection Vials
[0940] A solution of 100 g of the active ingredient according to the invention and 5 g of disodium hydrogen phosphate in 3 liters of double distilled water is adjusted to pH 6.5 using 2 N hydrochloric acid, sterile filtered, filled into injection vials and lyophilized under sterile conditions And sealed under sterile conditions. Each injection vial contains 5 mg of active ingredient.
Embodiment B
[0941] Embodiment B: Suppository
[0942] A mixture of 20 g of active ingredient according to the invention with 100 g of soy lecithin and 1400 g of cocoa butter is melted, poured into molds and allowed to cool. Each suppository contains 20 mg of active ingredient.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com