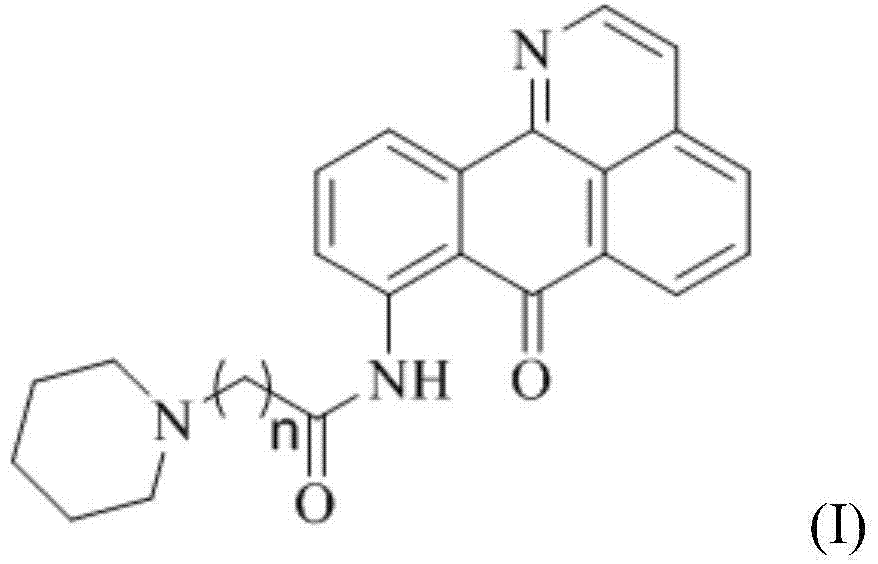
8-substitued oxoisoaporphine derivatives as well as synthetic method and application thereof
A technology of isoapofil and synthesis method, applied in the field of 8-substituted oxidized isoapofil derivatives and its synthesis, can solve problems that have not been seen before, and achieve good medicinal value and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
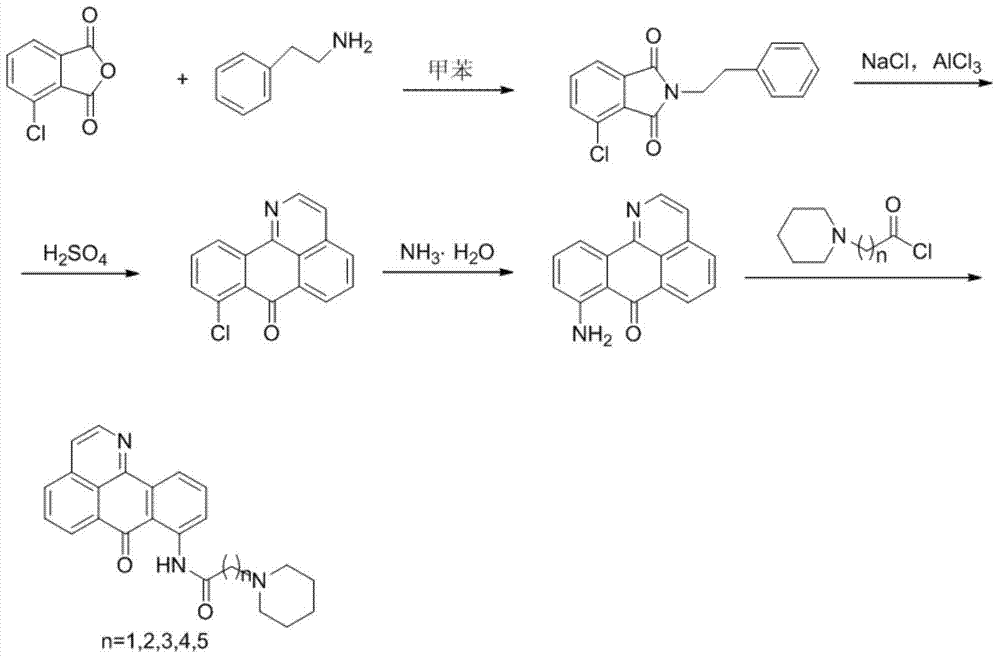
[0033] Example 1: Synthesis of 4-chloro-2-phenylisoindoline-1,3-dione (Compound 1)
[0034] Add 57g (0.1mol) of 3-chlorophthalic anhydride and 45g (0.1mol) of phenethylamine into a 1L three-hole round bottom flask, then add 500ml of toluene, reflux for 6 hours, pour out while it is hot, and cool. Crystals were separated out and filtered with suction to obtain compound 1 (white flake crystals) with a yield of about 98%.
[0035] Analyze compound 1 and its spectral characteristics are as follows:
[0036] 1 H NMR(CDCl 3 ,500MHz): δ3.00~3.04(m,2H),3.94~3.97(m,2H),7.23~7.34(m,5H),7.65(d,J=1.7,1H),7.66(s,1H) ,7.78(dd,J 1 =5.0and J 2 =3.2,1H);ESI-MS(m / z):287[M+H] + .
[0037] Therefore, it can be determined that the above compound 1 is 4-chloro-2-phenylisoindole-1,3-dione, and its structural formula is shown in the following formula:
[0038]
Embodiment 2
[0039] Example 2: Synthesis of 8-Cl-1-azabenzoanthrone (Compound 2)
[0040] Add 75g (0.56 mol) of anhydrous aluminum trichloride and 15g of sodium chloride into a 1L three-hole round bottom flask, mix and heat to 140℃ to melt, and then slowly add 53g (0.2mol) of compound 1 to it in batches After the addition is complete, the temperature is raised to 220°C, and the reaction is stirred for 3 hours. After being heated, it is poured into a mortar, cooled and mashed to obtain a reddish brown solid, which is placed in a drying vessel.
[0041] Take 600ml of concentrated sulfuric acid and add it to a 2L three-hole round bottom flask. When the temperature is raised to 80°C, the red-brown solid obtained above is added in batches. After the addition, the temperature is raised to 230°C, the reaction is stirred for 2.5 hours, cooled, and poured into In about 600 g of ice water, adjust the pH to about 2-3 with NaOH, precipitate a large amount of solids, filter with suction, and wash with water...
Embodiment 3
[0046] Example 3: Synthesis of 8-amino-7H-dibenzoquinolin-7-one (compound 3)
[0047] Take 1.5g of compound 2 in the inner pot of the autoclave, add 100ml of ammonia water, and react at 180°C for 12h, then stop the reaction. After cooling, adjust the pH to 7-8 with 3mol / L HCl, filter with suction, and dry. The crude product was purified by silica gel column chromatography (petroleum ether / chloroform=7:1) to obtain compound 3 (red solid) with a yield of 40%.
[0048] Analyzing compound 3, its spectral characteristics are as follows:
[0049] 1 H NMR (500MHz, DMSO): δ8.73(d,J=5.5Hz,1H), 8.45(d,J=7.2Hz,1H), 8.29(d,J=8.2Hz,1H), 8.06(d, J=8.4Hz,1H),8.02-7.97(m,1H),7.94(d,J=5.6Hz,1H),7.55-7.48(m,1H),7.00(d,J=7.3Hz,1H), 2.51(t,J=2.8Hz,2H);ESI-MS(m / z):247[M+H] + .
[0050] Therefore, it can be determined that the above compound 3 is 8-amino-7H-dibenzoquinolin-7-one, and its structural formula is shown in the following formula:
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com