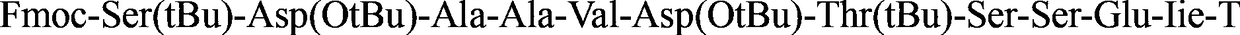Chemically modified thymosin α1 and its synthesis method
A technology of chemical modification and synthesis method, which is applied in the direction of chemical instruments and methods, thymosin, and peptide preparation methods. It can solve the problems of large molecular weight of PEG, difficulty in obtaining modified site products, and decreased biological activity of drugs, so as to achieve bioavailability. High, increased duration, extended half-life effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthetic method of the thymosin α1 of embodiment 1 chemical modification
[0039] Include the following steps:
[0040] (1) Weigh 200 mg of Fmoc-protected asparagine king resin (Fmoc-Asn(Trt)-WangResin 0.12 mmol / g) into a manual solid-phase peptide synthesizer, add DCM (dichloromethane) to swell for 30 minutes. Using Fmoc-protected amino acid raw materials, benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA) as peptide condensation Mixture, the equivalent ratio of resin and each protected amino acid and polypeptide condensing agent is tyrosine king resin: amino acid: HBTU: DIEA=1: 3: 3: 6, according to the standard Fmoc strategy synthesis Fmoc protects the king loaded with thymosin α1 Resin (Compound 1).
[0041] (2) After removing the terminal amino Fmoc protecting group with 2 ml of piperidine deprotecting agent (piperidine: DMF = 20:80 (v / v), dissolve 46 mg of myristic acid (0.2 mmol) in 2 ml Dimethylformamid...
Embodiment 2
[0046] The synthetic method of the thymosin α1 of embodiment 2 chemical modifications
[0047] Include the following steps:
[0048] (1) Weigh 200 mg of Fmoc-protected asparagine king resin (Fmoc-Asn(Trt)-WangResin 0.12 mmol / g) into a manual solid-phase peptide synthesizer, add DCM (dichloromethane) to swell for 30 minutes. Sequentially use Fmoc-protected amino acid raw materials (where the fourteenth position uses Fmoc-Lys(Dde)-OH), and use benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA) are polypeptide condensing agents, and the equivalent ratio of resin and each protected amino acid and polypeptide condensing agent is tyrosine king resin: amino acid: HBTU: DIEA=1: 3: 3: 6. Synthesize Fmoc-protected Wang resin loaded with thymosin α1 according to the standard Fmoc strategy. Subsequently, 2 ml of piperidine deprotecting agent (piperidine: DMF = 20: 80) was used to remove the Fmoc protecting group of the terminal amino g...
Embodiment 3
[0053] The synthetic method of the thymosin α1 of embodiment 3 chemical modifications
[0054] Include the following steps:
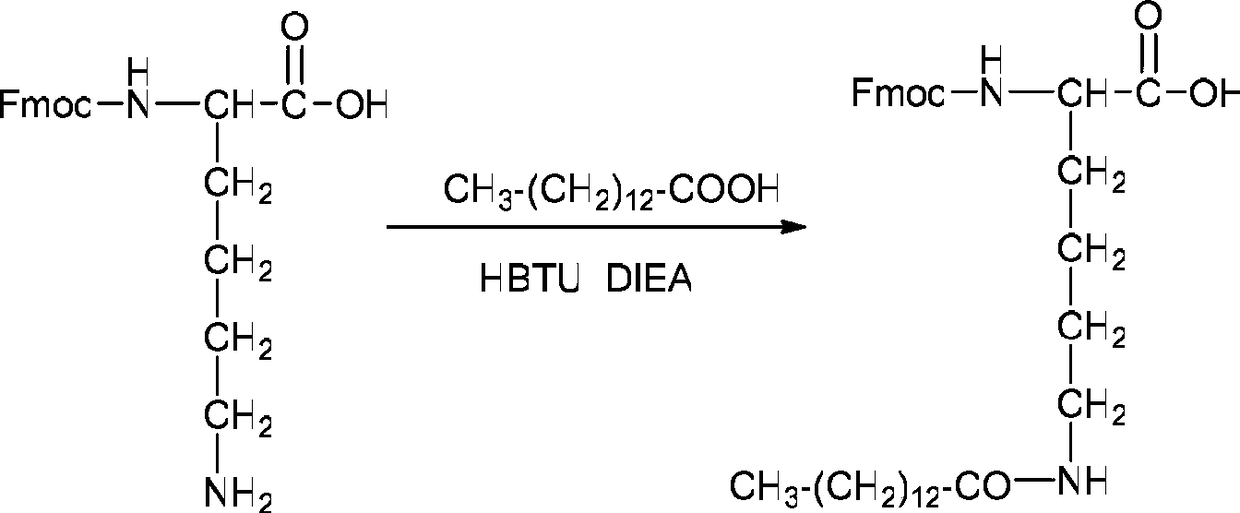
[0055] (1) Dissolve myristic acid (460 mg, 2 mmol) in 100 ml of dichloromethane (DCM), activate with equivalent HBTU (759 mg, 2 mmol) and DIEA (260 mg, 2 mmol) for 2 minutes, add Fmoc-Lys-OH (736 mg, 2 mmol), after reacting at room temperature for 1 hour, the DCM was evaporated under reduced pressure, and the crude product was purified by HPLC to obtain the lysine (Fmoc-Lys(Myristic acid) of the myristic acid modified side chain of Fmoc protection. )-OH) 872 mg, the yield was 76%. The reaction equation is as follows:
[0056]
[0057] (2) Weigh 200 mg of Fmoc-protected asparagine king resin (Fmoc-Asn(Trt)-WangResin 0.12 mmol / g) into a manual solid-phase peptide synthesizer, add DCM (dichloromethane) to swell for 30 minutes. Sequentially use Fmoc-protected amino acid raw materials (where the fourteenth position uses Fmoc-Lys(Myr)-OH), and use benzo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com