Preparation method of amorphous nickel sulfide
A nickel sulfide and amorphous technology, which is applied in the field of preparation of amorphous nickel sulfide, can solve the problems of increasing sodium sulfide dosage, decreasing utilization rate, large specific surface area, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
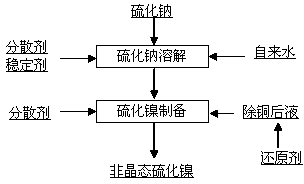
[0014] A kind of preparation method of amorphous nickel sulfide, concrete steps are as follows:
[0015] a, sodium sulfide dissolved
[0016] Add tap water at a temperature of 5~25°C to the sodium sulfide dissolving tank, add a stabilizer, the volume ratio of the stabilizer to tap water is 0.18~0.25:100, stir for 10~20 minutes, add industrial sodium sulfide with a purity of 60%, and dissolve Control the concentration of sodium sulfide at 90~120g / l, stir for 1~1.5 hours, then add dispersant, the volume ratio of dispersant to tap water is 0.15~0.2:100, stir for 0.5~1 hour;
[0017] b. Preparation of amorphous nickel sulfide
[0018] Take another container and add the solution after copper removal, the nickel concentration in the solution after copper removal is 75~85g / l, the temperature is 20~40°C, the pH value is 3.2~3.6, add anhydrous sodium sulfite, stir for 0.5~1 hour, after the sodium sulfite is dissolved The concentration is 0.8~1g / l, then add dispersant, the volume rati...
Embodiment 1
[0022] A kind of preparation method of amorphous nickel sulfide, concrete steps are as follows:
[0023] a, sodium sulfide dissolved
[0024] Add tap water at a temperature of 5~25°C to the sodium sulfide dissolving tank, add a stabilizer, the volume ratio of the stabilizer to tap water is 0.18:100, stir for 10~20 minutes, add industrial sodium sulfide with a purity of 60%, and dissolve the sodium sulfide Control the concentration at 90g / l, stir for 1-1.5 hours, then add dispersant, the volume ratio of dispersant to tap water is 0.15:100, stir for 0.5-1 hour;
[0025] b. Preparation of amorphous nickel sulfide
[0026] Take another container and add the solution after copper removal, the nickel concentration in the solution after copper removal is 75g / l, the temperature is 20~40°C, the pH value is 3.2~3.6, add anhydrous sodium sulfite, stir for 0.5~1 hour, the concentration of sodium sulfite after dissolution is 0.8g / l, then add dispersant, the volume ratio of dispersant to ...
Embodiment 2
[0028] A kind of preparation method of amorphous nickel sulfide, concrete steps are as follows:
[0029] a, sodium sulfide dissolved
[0030] Add tap water at a temperature of 5~25°C to the sodium sulfide dissolving tank, add a stabilizer, the volume ratio of the stabilizer to tap water is 0.25:100, stir for 10~20 minutes, add industrial sodium sulfide with a purity of 60%, and dissolve the sodium sulfide Control the concentration at 120g / l, stir for 1-1.5 hours, then add dispersant, the volume ratio of dispersant to tap water is 0.2:100, stir for 0.5-1 hour;
[0031] b. Preparation of amorphous nickel sulfide
[0032] Take another container and add the solution after copper removal, the nickel concentration in the solution after copper removal is 85g / l, the temperature is 20~40°C, the pH value is 3.2~3.6, add anhydrous sodium sulfite, stir for 0.5~1 hour, the concentration of sodium sulfite after dissolution is 1g / l, then add dispersant, the volume ratio of dispersant to co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com