A rapid hemostasis product for war wounds and its preparation method
A product, rapid technology, applied in the field of rapid hemostasis products, can solve problems such as insufficient activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
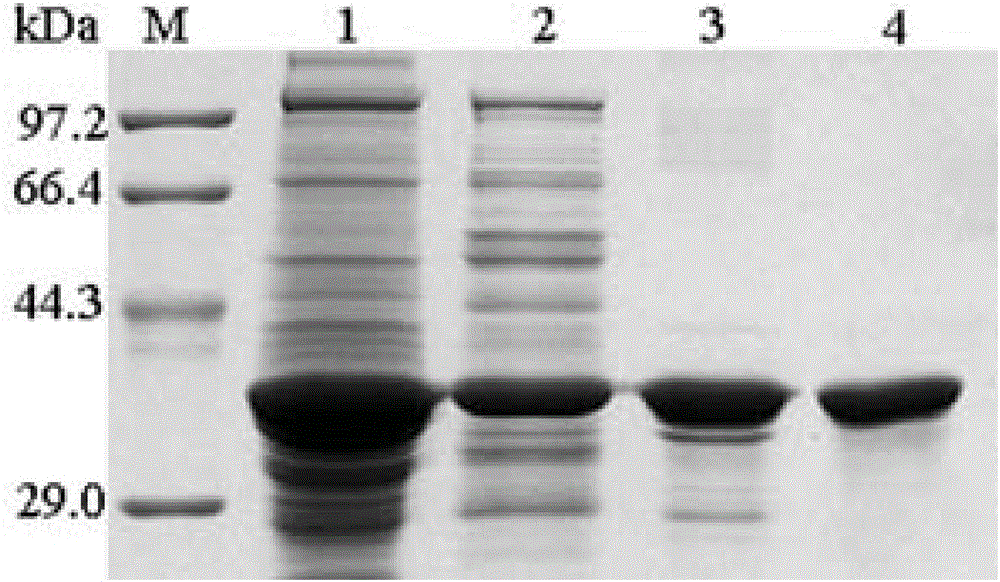
Embodiment 1
[0089] Example 1 Enzyme Activity Determination
[0090] 1) Preparation of reagents for measuring enzyme activity
[0091] Solution A (0.5L): Add 0.015mol (5.06g) of substrate Na-CBZ-Gln-Gly, 0.05mol (3.475g) of hydroxylamine hydrochloride, 0.005mol (1.536g) of reduced glutathione to 400ml of distilled water in a beaker After stirring with a magnetic stirrer for 20 minutes, add 0.1mol (12.11g) Tris, adjust the pH to 6.0 with 6mol / L (or 1mol / L) hydrochloric acid, transfer the solution to a 500ml volumetric flask, and wash the beaker with distilled water for 3 Pour it into a volumetric flask once, and set the volume to 500ml.
[0092] Solution B: 3mol / L HCl, 12% trichloroacetic acid (W / V), 5% ferric chloride (W / V, dissolved in 0.1mol / L hydrochloric acid, then filtered) mixed in a volume ratio of 1:1:1 .
[0093] 2) Determination of enzyme activity
[0094] Experimental group: Take 0.2ml of the sample to be tested, add 2ml of solution A and incubate at 37°C for 10 minutes, the...
Embodiment 2
[0095] The mutagenesis of embodiment 2 wild strains
[0096] The wild-type mTG strain is Streptomyces mobaraensis (such as the strain ATCC number 29032 of the US ATCC or the strain ATCC number 27441 of the US ATCC, or the strains of the China Microorganism Conservation Center such as CGMCC number 4.1719 and CGMCC number 4.5591).
[0097] Medium configuration: Gaoshi No. 1 medium: soluble starch 20g / L, KNO 3 1g / L, MgSO 4 ·7H 2 O0.5g / L, K 2 HPO 4 ·3H 2 O0.5g / L, NaCl0.5g / L, FeSO 4 ·7H 2 O0.01g / L, agar 20g / L, pH7.2-7.4. Fermentation medium: glycerin 20g / L, yeast extract 6g / L, fish meal peptone 25g / L, MgSO 4 ·7H 2 O2g / L, K 2 HPO 4 ·3H 2 O2g / L, pH7.4.
[0098] Add 10ml of cold sterile water to Gao's No. 1 medium, use an inoculation needle to fully scrape the surface hyphae, break up the spores, and filter with sterile filter paper.
[0099] The operation is carried out under red light or dark conditions, and the ultraviolet light is turned on 0.5 hours in advance to st...
Embodiment 3
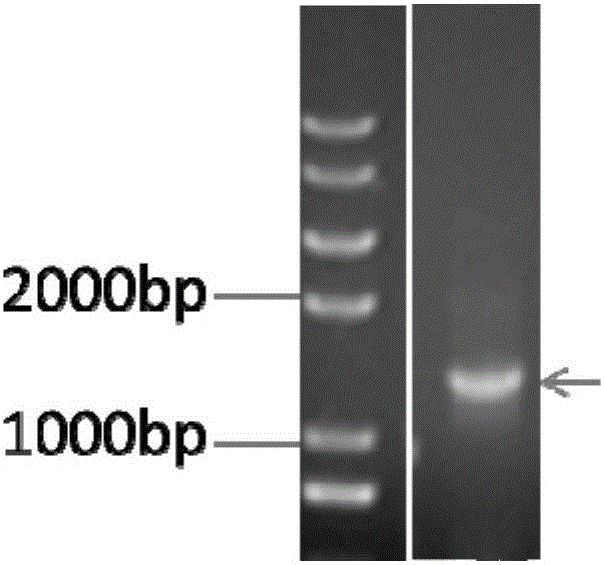
[0100] The acquisition of embodiment 3 mutant mTG gene
[0101]Genome extraction: Inoculate the fresh mycelium of the mutant strain with the highest enzyme activity in liquid culture in Example 2 into fresh medium, and cultivate for about 24 hours; collect 10 ml of thalline by centrifugation; fresh thalline in a mortar (-20 ℃ (pre-cooled), grind repeatedly with liquid nitrogen until fine powder, quickly and evenly distribute into two 1.5mL centrifuge tubes; add 550μL of TE buffer, add 30μL of 20% SDS solution preheated at 65℃, and vortex for 5 seconds , add 20 μL of 20 mg / mL proteinase K, mix gently, and incubate at 37°C for 1 hour; add an equal volume of Tris-saturated phenol / chloroform / isoamyl alcohol (25:24:1) for extraction, slightly invert to mix, and centrifuge at 10,000 rpm for 10 minutes ; Carefully draw the supernatant into a new centrifuge tube, add an equal volume of chloroform / isoamyl alcohol (24:1) for extraction, centrifuge at 10,000 rpm for 5 minutes; absorb the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com