Preparation method and kit of dendritic cell vaccine loaded with tumor-specific antigen epitope polypeptide
A tumor-specific antigen, dendritic cell technology, applied in anti-tumor drugs, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problems of cancer recurrence, poor prognosis, and inability to obtain patients, and achieve a radical cure for recurrence. and transfer, the effect of overcoming tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
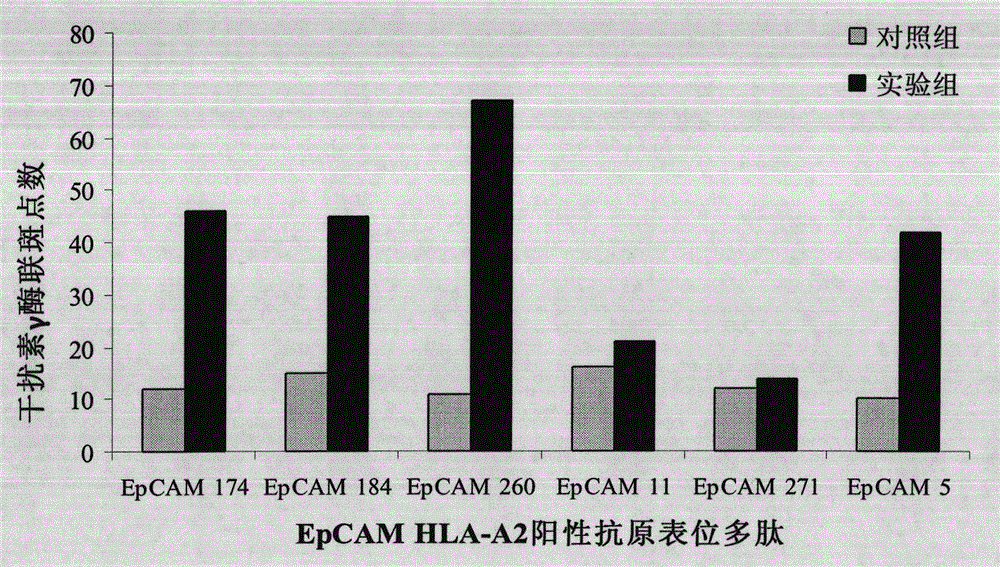
[0045] Preparation Example 1 Prediction of Human Epithelial Cell Adhesion Molecule HLA-A2010 Positive Epitope Polypeptide
[0046] Using the Bioinformatics and Molecular Analysis System (BIMAS) to predict the binding of HLA peptides, screen the HLA epitope peptides that specifically bind to human epithelial cell adhesion molecules, and select 6 amino acid sequences that have a binding affinity of more than 100 to HLA-A0201 , see Table 1:
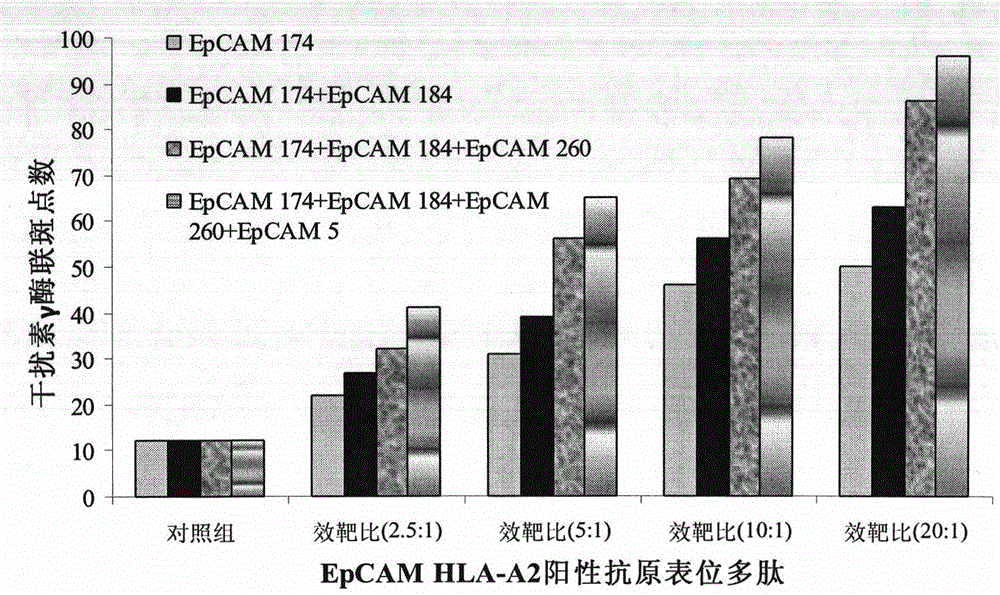
[0047] ranking start site Peptide Amino Acid Sequence binding affinity score 1 174 YQLDPKFIT 125.377 2 184 ILYEN NVIT 68.146 3 260 SMQGLKAGV 50.232 4 11 LLLAAATAT 46.873 5 271 VIVVVVIAV 37.393 6 5 QVLAFGLLL 26.281
preparation example 2
[0048] Preparation Example 2 Dendritic Cell Acquisition
[0049] Collect 2-4 liters of peripheral blood from tumor patients with a blood cell separator and purify the mononuclear cells with the lymphocyte separation medium; after counting by the cell counter, the mononuclear cells are diluted to 3-5×10 with AIM-V medium 6 , add 75cm 2 Attached to the wall in the culture bottle;
[0050] At 37°C, 5% CO 2 After incubation in the incubator for 90 minutes, non-adherent cells were washed and collected for activating T lymphocytes; adherent cells were induced by adding complete AIM-V medium, containing 5% autologous serum, 1000IU / mL recombinant human granules Macrophage colony-stimulating factor and 500IU / mL recombinant human interferon-α;
[0051] Harvest immature dendritic cells (DCs) on the fourth day of culture; mature DCs were induced to the fifth day with complete AIM-V medium, containing 5% autologous serum, 1000IU / mL recombinant human granulocyte macrophage colonies Stim...
Embodiment 3
[0052] Example 3 EpCAM epitope polypeptide-loaded DCs
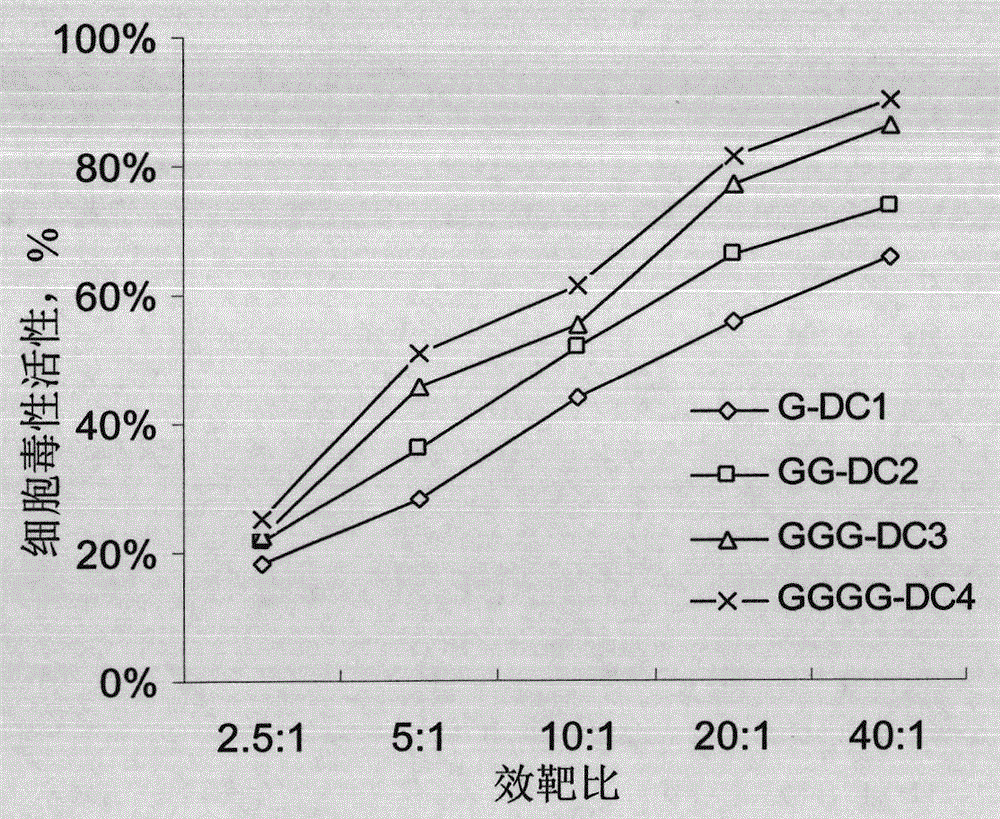
[0053] Cultured to the fourth day, 3×10 6 An immature DCs with epithelial cell adhesion molecule protein SEQ ID NO.1-6 more than one epitope polypeptide composition at 37 ° C, 5% CO 2 Load in the incubator for 2-4 hours; induce culture with full AIM-V medium until the fifth day, and harvest the mature antigen polypeptide-loaded DCs by centrifugation (10min, 1000rpm); the obtained DCs are washed 3 times with normal saline, and then resuspended into normal saline to a concentration of 5×10 6 mature DC / mL, and add human albumin with a final concentration of 1% by mass to volume to prepare a DC vaccine loaded with the EpCAM antigen epitope polypeptide composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com