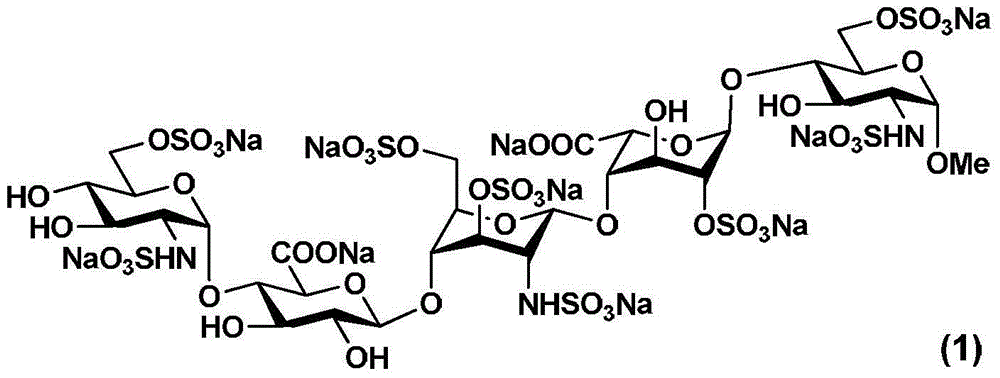
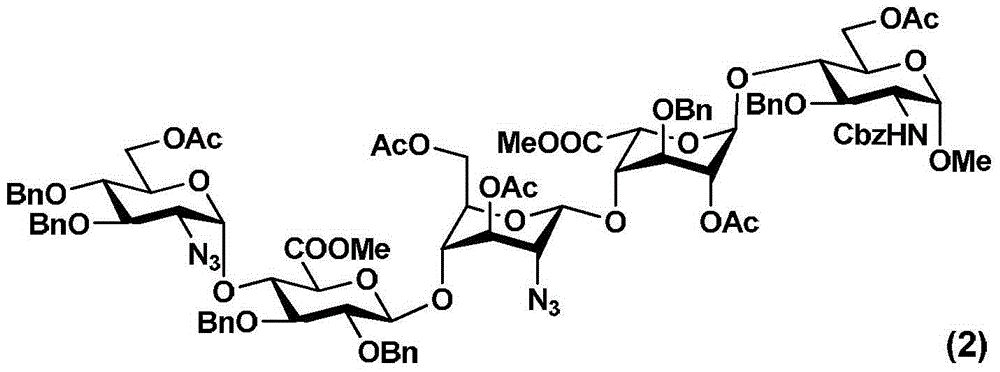
Fondaparinux sodium pentasaccharide intermediate and preparation method thereof
An organic solvent and catalyst technology, applied in the field of chemical preparation, can solve the problems of low yield of pentasaccharide intermediates and difficult separation of isomers, and achieve the effects of reducing the difficulty of synthesis, easy purification, and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
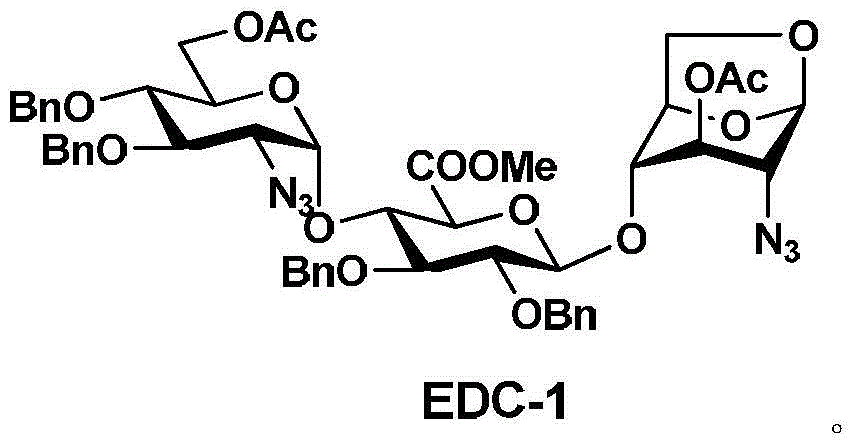
[0051] 1. The preparation route of trisaccharide (EDC-1):
[0052] Monosaccharide E-3 and disaccharide DC-2 were dissolved in an organic solvent, and the trisaccharide EDC-1 was obtained by coupling in the presence of a catalyst. The synthetic route is as follows:
[0053]
[0054] 2. Preparation route of trisaccharide (EDC-4):
[0055] Step 1, in the presence of an organic solvent, the trisaccharide is treated with acetic anhydride and Lewis acid to obtain the trisaccharide EDC-2;
[0056] Step 2, in the presence of an organic solvent, the trisaccharide EDC-2 is treated with an organic base to obtain EDC-3;
[0057] Step 3, in the presence of an organic solvent, trisaccharide EDC-3 is treated with trichloroacetonitrile to obtain trisaccharide EDC-4.
[0058] The synthetic route is as follows:
[0059]
[0060] 3. Preparation route of pentasaccharide (EDCBA-1):
[0061] In the presence of organic solvent and catalyst, trisaccharide EDC-4 and disaccharide BA-2 w...
Embodiment 1、 3
[0063] Embodiment 1, the preparation of trisaccharide (EDC-1);
[0064] 200 grams of compound (DC-2) and 205 grams of compound (E-3) were dissolved in 3.5 liters of dichloromethane, cooled to -50 ° C, added 30 grams of silver trifluoromethanesulfonate, reacted for 3 hours; concentrated under reduced pressure, 220 g of trisaccharide (EDC-1) were obtained by recrystallization and purification; the yield of the obtained product was: 65.5%.
[0065] 1H NMR (400MHz, CDCl3): δ2.03 (s, 3H), 2.10 (s, 3H), 3.20 (d, 1H), 3.26 (dd, 1H), 3.51 (t, 1H), 3.55~3.67 (m , 3H), 3.75(s, 3H), 3.77(dd, 2H), 3.88(t, 1H), 3.99(dd, 2H), 4.15(t, 1H), 4.23(d, 2H), 4.55(m, 2H), 4.71(dd, 2H), 4.82(t, 2H), 4.86(d, 2H), 5.02(dd, 2H), 5.21(d, 1H), 5.47(d, 1H), 5.53(d, 1H ), 7.22~7.37 (m, 20H).
[0066] ESI / MS+(m / z): 1031.9 [M+23=1031.4].
[0067] The structural formula of the trisaccharide EDC-1 is:
[0068]
Embodiment 2、 3
[0069] Embodiment 2, preparation of trisaccharide (EDC-1);
[0070] 200 grams of compound (DC-2) and 205 grams of compound (E-3) were dissolved in 3.5 liters of dichloromethane, cooled to 0 ° C, added 30 grams of silver trifluoromethanesulfonate, reacted for 3 hours; concentrated under reduced pressure, and weighed Crystallization and purification yielded 245 grams of trisaccharide (EDC-1); the yield of the obtained product was: 72.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com