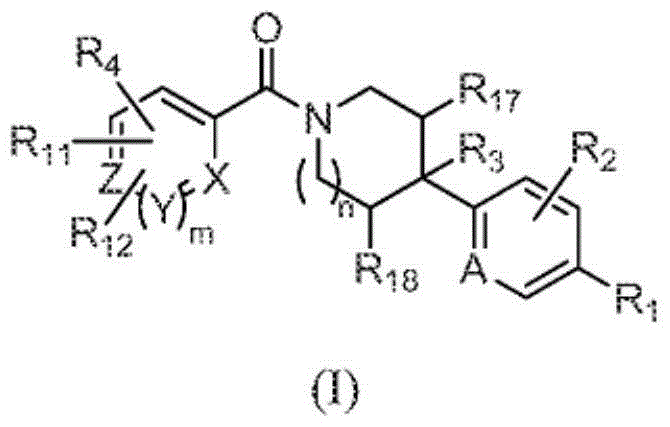
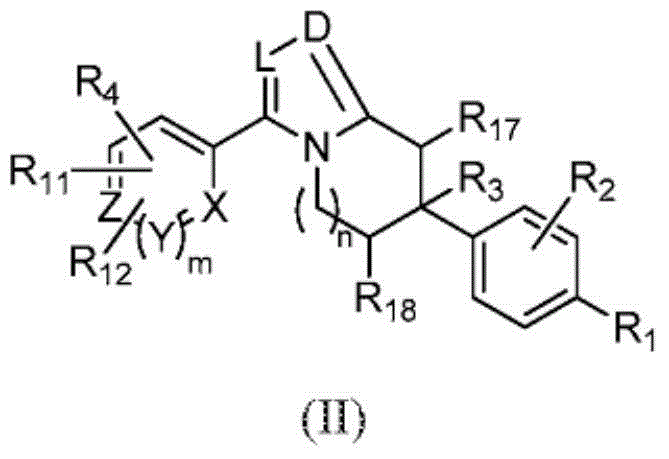
Heterocyclic modulators of lipid synthesis
A compound and cycloalkyl technology, applied in the field of heterocyclic regulators of lipid synthesis, can solve problems such as anemia, weakened nutrient absorption, impaired immune function, destruction, etc., and achieve anti-viral and anti-cancer therapy, improved anti-cancer Effects of virus and anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[2528] Example 1-FASN inhibition by compounds of the present disclosure
[2529] Determination of FASN biochemical activity: FASN enzyme was isolated from SKBr3 cells. SKBr3 is a human breast cancer cell line with high FASN expression levels. It is expected that FASN contains approximately 25% cytosolic protein in this cell line. SKBr3 cells were homogenized in a Dounce homogenizer and then centrifuged at 4°C for 15 minutes to remove particulate matter. The supernatant was then analyzed for protein content, diluted to an appropriate concentration and used to measure FASN activity. The presence of FASN was confirmed by immunoblotting analysis. A similar method for isolating FASN from SKBr3 cells is described in Teresa, P. et al. (Clin. Cancer Res. 2009; 15(24), 7608-7615).
[2530] The FASN activity of SKBr3 cell extracts was determined by measuring the amount of thiol-containing coenzyme A (CoA) released during NADPH oxidation or during the fatty acid synthase reaction. The d...
Embodiment 2
[2531] Example 2-Antiviral activity
[2532] Use the HCV 1b replication system to evaluate the antiviral activity of the structure (I-Z):
[2533]
[2534] The replicon was constructed using the ET (luc-ubi-neo / ET) cell line, which is an HCV replicon with a stable luciferase (Luc) reporter and Three cell cultures were adapted to mutant Huh7 human liver cancer cell lines (Pietschmann et al. (2002) J. Virol. 76: 4008-4021). The HCV replicon antiviral evaluation analysis investigated the effect of the compound at six half-log concentrations. Human interferon alpha-2b was included in each operation as a positive control compound. The near-confluent culture of the ET line was plated out in a 96-well plate dedicated to the analysis of cell number (cytotoxicity) or antiviral activity, and the drug was added to the appropriate well the next day. When the cells are still near confluence after 72 hours, the cells are processed. Determine EC 50 (The replicon was inhibited by 50% and 90% ...
Embodiment 3
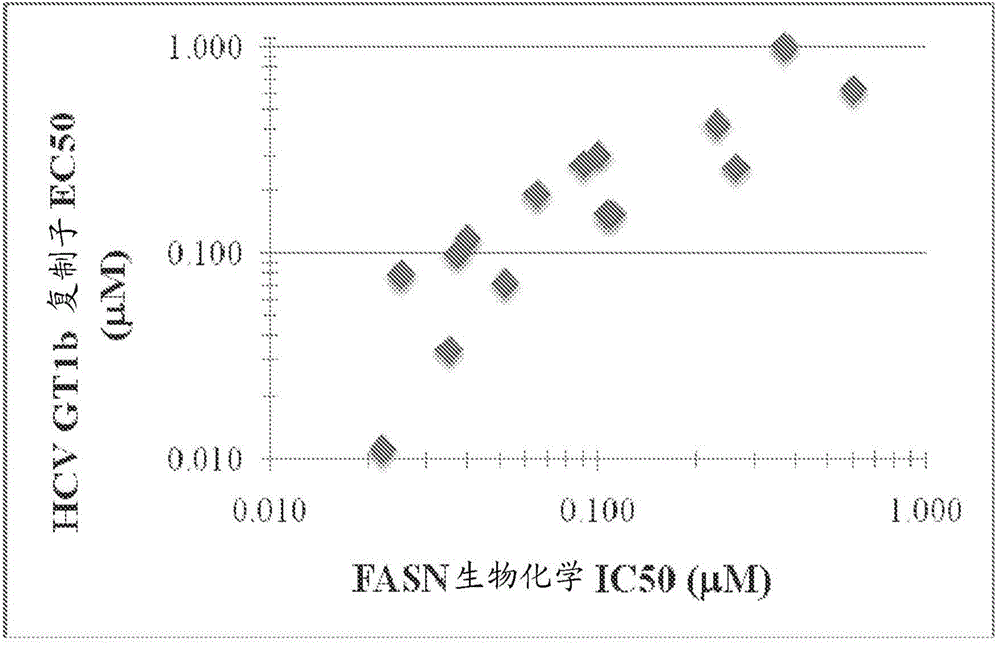
[2537] Example 3-FASN inhibition is related to HCV inhibition
[2538] The HCV replicon system was used to measure the antiviral activity of 15 compounds of the present disclosure (the numbers correlate with the compounds in Table 1). According to published methods (Lohmann et al., (1999) Science 285(5424): 110-113; Lohmann et al., (2001) J. Virol 75(3): 1437-1449 and Qi et al., (2009) Antiviral Res. 81(2):166-173), using Huh7 to establish a replicon cell line 1b (HCV 1b / Luc-Neo replicon (1b Con1 integrated with firefly genes)) by G418 selection. Use synthetic gene fragments to assemble the replicon. GT1b has PV-EKT and has 3 adaptive mutations E1202G (NS3), T1280I (NS3), K1846T (NS4B) and the backbone is Con1. The medium is:
[2539] a) DMEM supplemented with 10% FBS, G418 (250μg / ml), streptomycin (100μg / ml) / penicillin (100U / ml), L-glutamine (100×), NEAA (100×)
[2540] b) The culture medium is prepared as follows:
[2541] i) 500ml DMEM medium (Gibco, catalog number 11960-077) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com