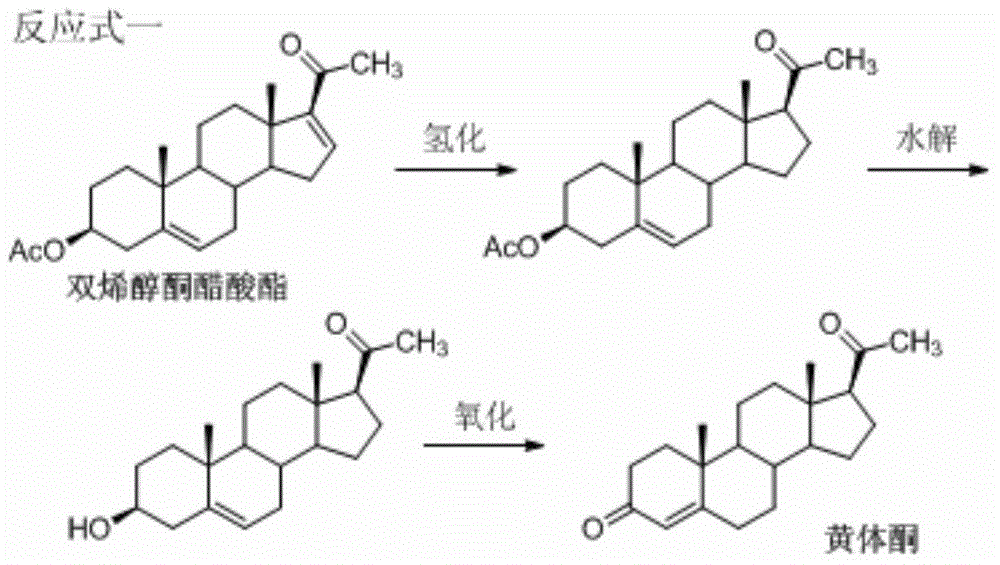
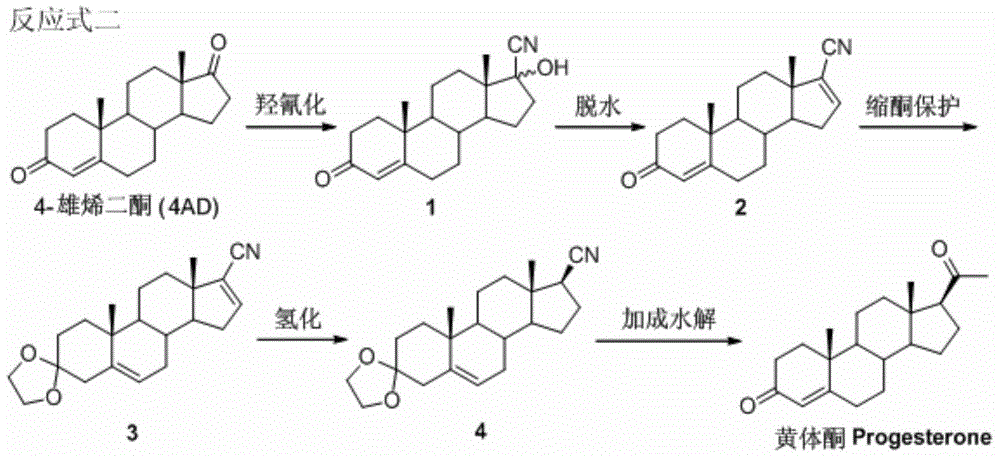
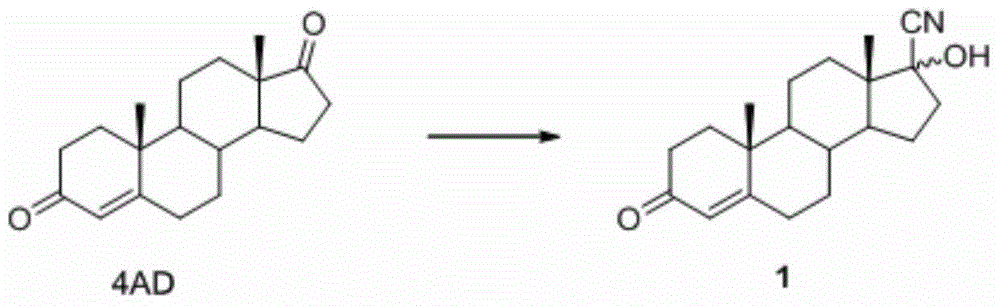
A kind of method for preparing progesterone
A technology of progesterone and androstenedione, which is applied in the fields of steroids and organic chemistry, can solve the problems of progesterone synthesis cost increase and labor cost increase, and achieve high production application and economic value, simple circuit and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Put 30g of raw material 4AD, 30mL of methanol, 45mL of acetone cyanohydrin into the reaction vessel, raise the temperature to dissolve, add potassium carbonate aqueous solution (1.0g of potassium carbonate + 100mL), and keep the reaction at 30-50°C for about 20 hours. Cool the reaction solution to 0-10°C, keep it warm for 30 minutes, filter and flush with water, soak the filter cake in 10% dilute hydrochloric acid for 0.5 hours, filter to obtain the crude product, and refine 17-hydroxycyano-4-androsten-3-one (compound 1), the yield is about 95% (m / m); the product is a mixture of isomers.
[0035] EI MS (70eV, m / z): 313 (M + ,75%).
Embodiment 2
[0037]
[0038]Add 5g of compound 1, 25mL of pyridine, stir and add 5mL of phosphorus oxychloride, raise the temperature to 115°C, reflux for 30 minutes, TLC shows that the reaction is complete, lower the temperature below 10°C, slowly add water dropwise until it is completely dissolved, concentrate under reduced pressure, and water analysis, Filter and flush water to neutral to get wet product. Then dissolve it with dichloromethane, separate the water layer, filter, concentrate under reduced pressure to dryness, crystallize methanol, freeze, filter a small amount of ice methanol, rinse, and dry to obtain 17-cyano-4,16-androstadiene- 3-Keto (compound 2), 3.2 g. The yield is about 65% (m / m). EI MS (70eV, m / z): 295 (M + ,80%); 1 H NMR (300MHz, CDCl 3 )δ6.61(m,1H,16-H),5.71(s,1H,4-H),1.20(s,3H,19-H),0.95(s,3H,18-H); 13 C NMR (100MHz, CDCl 3 )δ199.3, 170.1, 147.3, 127.2, 124.2, 115.7, 55.3, 53.9, 48.1, 38.7, 35.6, 34.1, 33.9, 33.8, 32.8, 32.5, 31.6, 20.6, 17.2, 16.3.
Embodiment 3
[0040]
[0041] Add 5g of compound 2, 4.3mL of ethylene glycol, 3mL of triethyl orthoformate and 30mL of dichloromethane into the reaction vessel, then add the catalyst p-toluenesulfonic acid 0.29g, react at 35-45°C for 8-12 hours, and the reaction is complete Afterwards, the reaction solution was neutralized with an alkaline solution at 0-5°C, concentrated, water-analyzed, filtered, and the filter cake was washed with water until neutral recrystallization, and dried to obtain 3,3-ethylenedioxy-17-cyano-4, 4.25 g of 16-androstadiene (compound 3), the yield is about 85% (m / m). EI MS (70eV, m / z): 339 (M + ,78%); 1 H NMR (300MHz, CDCl 3 )δ 6.61(m,1H,16-H),5.34(m,1H,6-H),3.94(m,4H,-OCH 2 CH 2 O-),1.05(s,3H,19-H),0.93(s,3H,18-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com