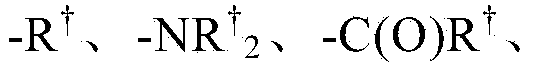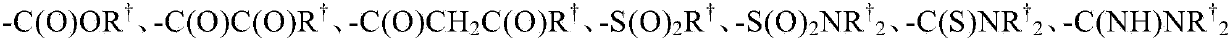Pi3 kinase inhibitors and uses thereof
A technology of unsaturated compounds, applied in the fields of application, biocides, anti-inflammatory agents, etc., can solve the problems of unidentified class II PI3K regulatory subunits, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0717]
[0718] 1-(4-(4-(2-(2-aminopyrimidin-5-yl)-6-morpholinopyridin-4-yl)phenyl)piperazin-1-yl)-6-methylheptyl- 5-ene-1,4-dione (I-1). The title compound was synthesized according to the procedure described below.
[0719]
[0720] Step 1a: 4-(6-Chloro-4-iodopyridin-2-yl)morpholine (Intermediate 1a)
[0721]
[0722] 2,6-Dichloro-4-iodopyridine (2.0 g, 7.3 mmol), morpholine (700 uL, 8.0 mmol) and 1.5 mL of DIPEA were heated in 15 mL of anhydrous dioxane at 120° C. for 24 hours. After concentration and the usual aqueous work-up with ethyl acetate-water, the reaction mixture was column chromatographed on silica gel eluting with heptane / ethyl acetate (v / v 6 / 1) to obtain 1.74 g of the desired product as white crystals. MS: m / z 325.0 (ES+).
[0723] Step 1b: tert-butyl 4-(4-(2-chloro-6-morpholinylpyridin-4-yl)phenyl)piperazine-1-carboxylate (Intermediate 1b)
[0724]
[0725] Under Ar, intermediate 1a (97 mg, 0.3 mmol), 4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborola...
example 2
[0733]
[0734] N-(4-((2-(2-aminopyrimidin-5-yl)-6-morpholinopyridin-4-yl)ethynyl)phenyl)-6-methyl-4-oxohept-5 - enamide (I-2). The title compound was synthesized according to the following intermediates and the procedure described below.
[0735]
[0736] Step 2a: N-(4-((2-Chloro-6-morpholinopyridin-4-yl)ethynyl)phenyl)-6-methyl-4-oxohept-5-enamide (intermediate 2a)
[0737]
[0738] Intermediate 1a (55 mg, 170 umol), N-(4-ethynylphenyl)-6-methyl-4-oxohept-5-enamide (44 mg, 170 umol, readily available from 4 -ethynylaniline and 6-methyl-4-oxohept-5-enoic acid), PdCl 2 (PPh 3 ) 2 (6 mg), CuI (15 mg), 100 uL DIPEA in 2 mL DMA heated at 80° C. overnight. After working up with ethyl acetate and water, the reaction mixture was column chromatographed on silica gel eluting with heptane / ethyl acetate (v / v 3 / 2) to obtain 55 mg of the desired product as a white solid. MS: m / z 452.1 (ES+).
[0739] Step 2b: N-(4-((2-(2-aminopyrimidin-5-yl)-6-morpholinopyridin-4-yl)ethyn...
example 3
[0743] The following compounds belong to the general structures shown in the table below, which were used in Step 1b in a manner similar to that described in Example 1 using 4-(4,4,5,5-tetramethyl-1,3,2-di Oxaborolan-2-yl)-5,6-dihydropyridine-1(2H)-carboxylic acid tert-butyl ester to synthesize.
[0744]
[0745] (a pair of conformers from tetrahydropyridine)
[0746]
[0747]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com