Fluorazame compound with herbicidal activity and preparation method thereof
A technology of fenflufen and a compound, which is applied to fenflufen with herbicidal activity and the field of preparation thereof, can solve problems such as unsatisfactory control effect, and achieve environmental friendliness, high yield and good purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
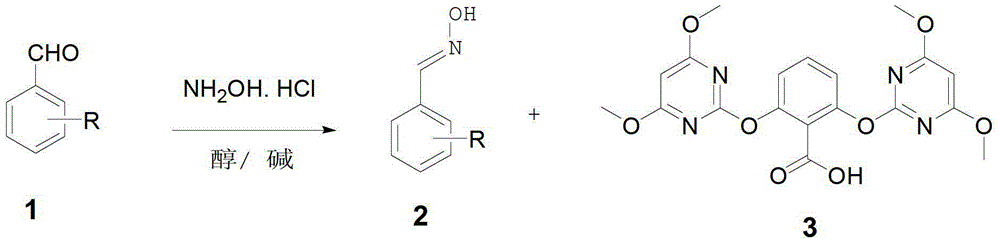
[0120] Below in conjunction with specific embodiment, the present invention is further described in detail:
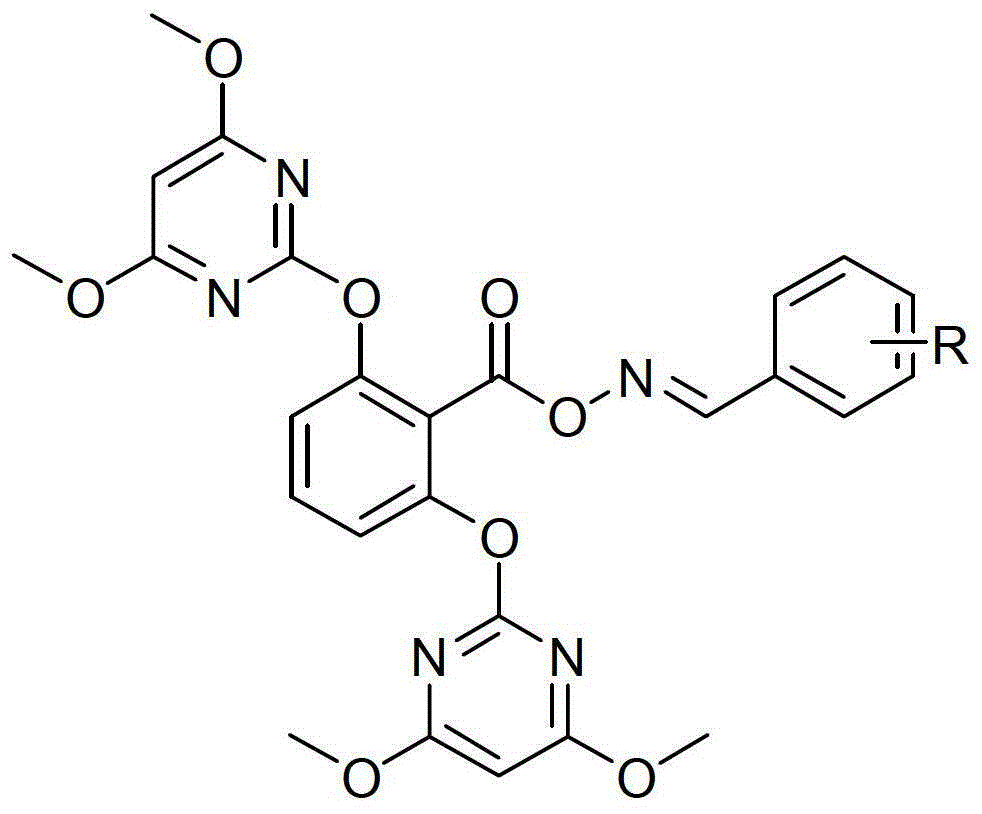
[0121] The to-be-prepared herbicide containing trifluoromethylbenzene-trifluoromethyl benzene-containing fluramiflub compound with high herbicidal activity is represented by the general formula (I):
[0122]
[0123] R=o,m,p-CF3
[0124] Wherein R is a substituted compound of o-, m-, p-trifluoromethylbenzene.
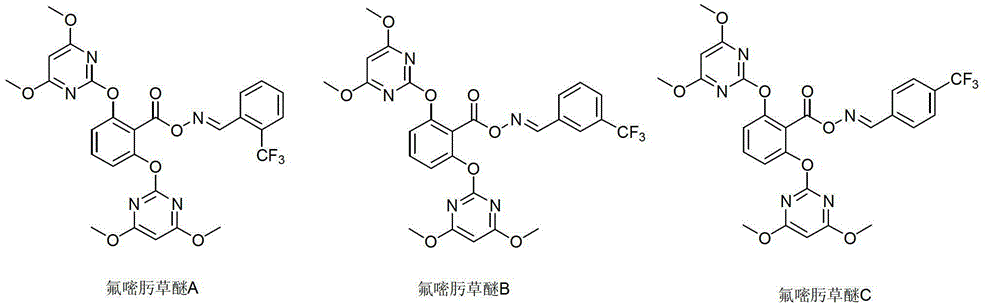
[0125] There are three structures of fluramifluzed compounds:
[0126] Fluorumazepam A: O-[2,6-bis(4,6-dimethoxypyrimidinyl-2-yloxy)benzoyl](2-trifluoromethyl)benzaldehyde oxime;
[0127] Fluoxaflub B: O-[2,6-bis(4,6-dimethoxypyrimidinyl-2-yloxy)benzoyl](3-trifluoromethyl)benzaldehyde oxime;
[0128] Fluorumazepam C: O-[2,6-bis(4,6-dimethoxypyrimidinyl-2-yloxy)benzoyl](4-trifluoromethyl)benzaldehyde oxime;
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com