Preparation method of sitagliptin and intermediate of sitagliptin
A compound and organic acid technology, applied in the field of a method of drug sitagliptin and its intermediates, can solve the problems of limited induction ability of R-(+)-phenylethylamine, loss of isomers, etc. Strong sex-inducing ability, easy to operate, and low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
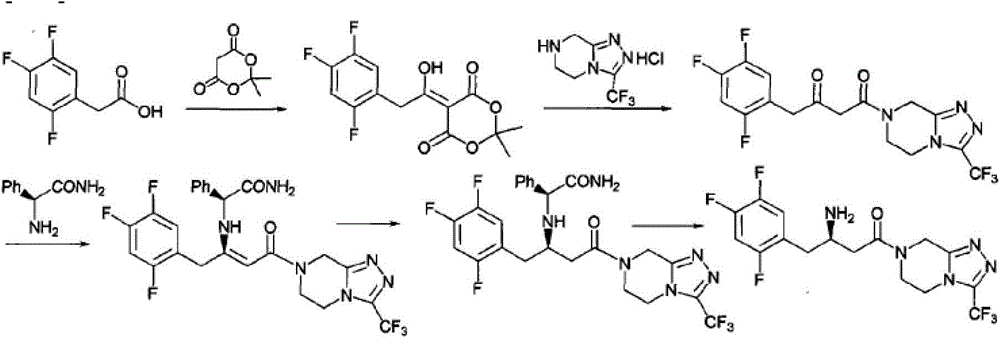
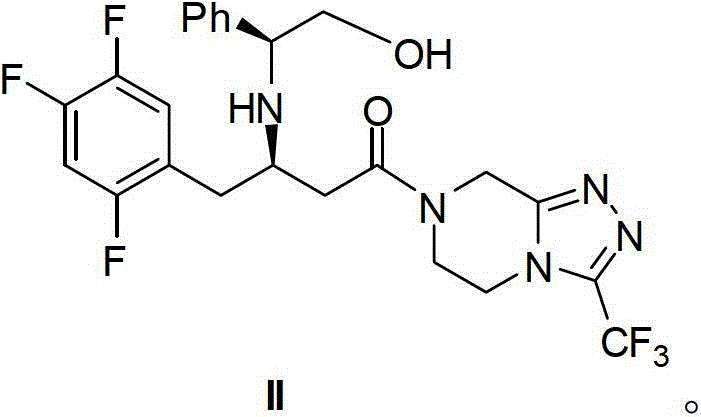
[0044] Dissolve 2.5 g of compound III in 12 mL of methanol, and stir to dissolve. 0.8 g of compound IV and 0.2 g of acetic acid were added to the system. After the addition is completed, the temperature is raised to 60-65° C. to react for about 8 hours, and the reaction is detected by TLC until the conversion of the raw materials is basically complete.
[0045] Heating was stopped, the temperature of the reaction system was lowered to 0-10°C, and stirring was continued for 1 hour. Filter and wash the filter cake with 12 mL of methanol. The obtained solid was dried in vacuo to obtain 2.8g of compound I with a yield of 86% and a purity of 98%. 1 H NMR (400MHz, DMSO-d 6 ):δ10.159-10.181(d,1H),7.437-7.488(m,1H),7.220-7.288(m,2H),7.187-7.208(q,2H),7.115-7.136(t,2H),5.075 -5.101(q,1H),4.977(s,1H),4.806(s,2H),4.461-4.482(t,1H),4.160-4.185(t,2H),3.934(s,2H),3.405-3.583 (m,4H).
preparation Embodiment 2
[0047] 5 g of Compound III was dissolved in 25 mL of ethanol, and stirred to dissolve. 1.6 g of compound IV and 0.4 g of acetic acid were added to the system. After the addition is completed, the temperature is raised to 60-65° C. to react for about 8 hours, and the reaction is detected by TLC until the conversion of the raw materials is basically complete.
[0048] Heating was stopped, the temperature of the reaction system was lowered to 0-10°C, and stirring was continued for 1 hour. Filter and wash the filter cake with 25 mL of ethanol. The obtained solid was dried in vacuo to obtain 5.5 g of compound I with a yield of 84% and a purity of 97%.
preparation Embodiment 3
[0050] 23 g of compound III was dissolved in 115 mL of isopropanol, and stirred to dissolve. 8.4 g of compound IV and 1.7 g of acetic acid were added to the system. After the addition is completed, the temperature is raised to 60-65° C. to react for about 8 hours, and the reaction is detected by TLC until the conversion of the raw materials is basically complete.
[0051] Heating was stopped, the temperature of the reaction system was lowered to 0-10°C, and stirring was continued for 1 hour. Filter and wash the filter cake with 12 mL of isopropanol. The obtained solid was dried in vacuo to obtain 25g of compound I with a yield of 81% and a purity of 98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com