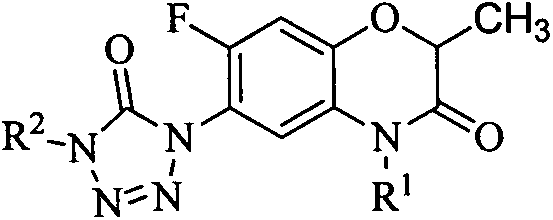
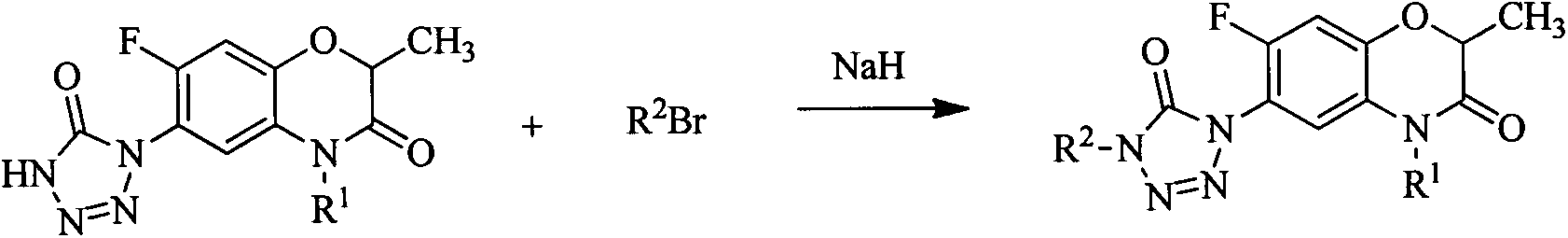Herbicidal activity of tetrazoleone derivative containing 7-fluoro-2-methyl benzoxazine-3-one structure
A technology of methylbenzoxazine and herbicidal activity, which is applied in the field of herbicidal activity of tetrazolone derivatives, and can solve the problems of no specific records, no specific records of compound herbicidal activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
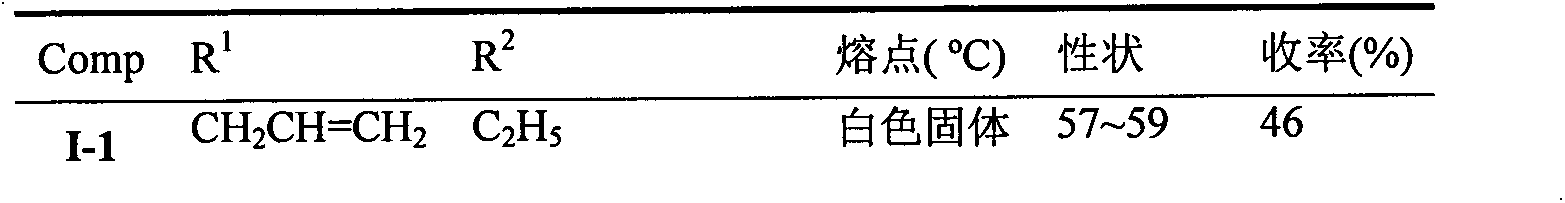
[0018] Example 1: 1-ethyl-4-(7-fluoro-2-methyl-4-allylbenzoxazin-3-one-6-yl)tetrazolone (I-1)
[0019] Take 4mmol of 4-(7-fluoro-2-methyl-4-allylbenzoxazin-3-one-6-yl)tetrazolone and 6mmol of bromoethane into a 50mL two-necked bottle, add 30mL of THF, and stir to dissolve Then add 20mmol triethylamine, reflux for 8h. The reaction solution was poured into an aqueous solution, and the organic phase was separated and dried. The pure product was obtained by column chromatography.
Embodiment 2
[0020] Embodiment 2: 1-(3-chloropropyl-1)-4-(7-fluoro-2-methyl-4-allyl benzoxazin-3-one-6-yl)tetrazolone ( I-2)
[0021] Take 3.5mmol of 4-(7-fluoro-2-methyl-4-allylbenzoxazin-3-one-6-yl)tetrazolone and 5.2mmol of 1-bromo-3-chloropropane into a 50mL two-necked bottle, Add 30mLTHF, stir to dissolve, then add 22mmol triethylamine, and reflux for 7.4h. The reaction solution was poured into an aqueous solution, and the organic phase was separated and dried. The pure product was obtained by column chromatography.
Embodiment 3
[0022] Example 3: 1-ethyl-4-(7-fluoro-2,4-dimethylbenzoxazin-3-one-6-yl)tetrazolone (I-3)
[0023] Take 2.4mmol of 4-(7-fluoro-2,4-dimethylbenzoxazin-3-one-6-yl)tetrazolone and 4.3mmol of bromoethane into a 50mL two-necked bottle, add 33mL of THF, and stir to dissolve Then add 20mmol triethylamine, reflux for 5.9h. The reaction solution was poured into an aqueous solution, and the organic phase was separated and dried. The pure product was obtained by column chromatography.
[0024] Compound (I) was synthesized according to a similar method, and the physical parameter results of the compound are shown in Table 1.
[0025] The physical property characterization of compound (I) of table 1
[0026]
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com