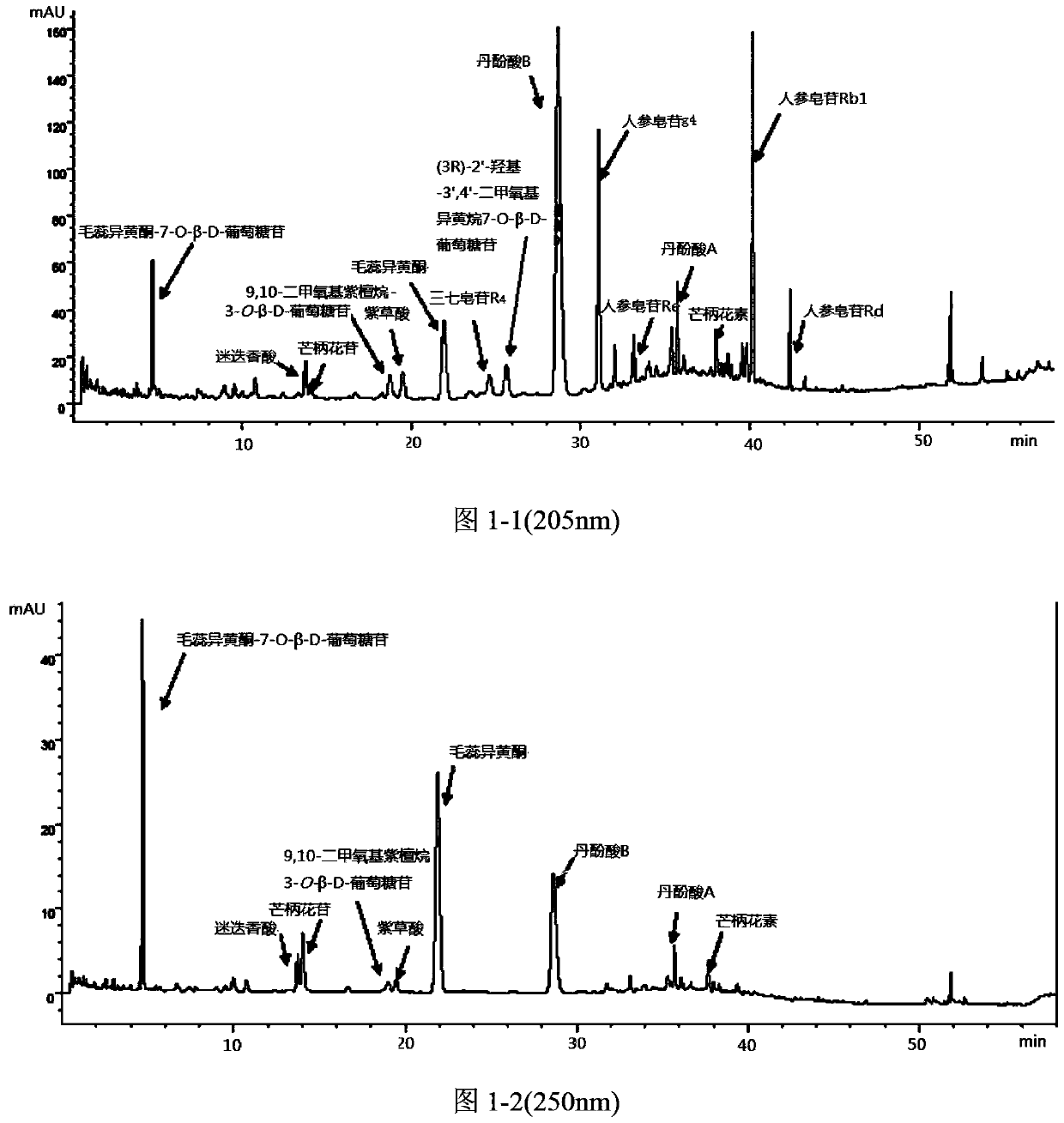
Pharmaceutical composition for prevention and treatment of chronic glomerular diseases and preparation method of pharmaceutical composition
A composition and technology of glomerulonephritis, applied in the direction of drug combination, urinary system diseases, medical preparations containing active ingredients, etc., to achieve the effect of alleviating renal fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] formula:
[0058] 27 parts of Astragalus, 9 parts of Panax notoginseng and 18 parts of Danshen;
[0059] Preparation:
[0060] (1) Prescription quantity Astragalus medicinal material was added with 8 times the weight volume concentration of 60% ethanol for reflux extraction for 3 hours and extracted 3 times to obtain Astragalus extract. The Radix Notoginseng medicinal material was refluxed and extracted by adding 8 times of ethanol with a weight volume concentration of 80% for 3 hours, and extracted 3 times to obtain the Radix Notoginseng extract. Salvia miltiorrhiza medicinal material was added with 6 times the weight volume concentration of 50% ethanol for reflux extraction for 3 hours and extracted 3 times to obtain the salvia miltiorrhiza extract. The extracts were decompressed to recover ethanol, and concentrated to 1.8±0.5g·mL based on the amount of medicinal materials -1 concentration.
[0061] (2) The extracts are respectively absorbed by the macroporous res...
Embodiment 2
[0078] formula:
[0079] 18 parts of Astragalus, 6 parts of Panax notoginseng and 6 parts of Danshen;
[0080] Preparation:
[0081] Mix the medicinal materials of Astragalus membranaceus, Panax notoginseng and Salvia miltiorrhiza in the prescribed amount, then add 10 times of ethanol with a weight volume concentration of 80% to reflux for extraction for 4 hours, extract twice, recover ethanol from the extract under reduced pressure, and concentrate to 1.8±0.3g based on the amount of medicinal materials ·mL -1 Concentration, to obtain the extract;
[0082] (2) Adsorb the extract with a macroporous resin, first elute impurities with water 5 times the weight of the macroporous resin, and then elute impurities with 10% ethanol at a volume concentration 4 times the weight of the macroporous resin;
[0083] (3) Elute with 75% ethanol at a volume concentration 10 times the weight of the macroporous resin, recover ethanol from the eluate under reduced pressure, concentrate, and dr...
Embodiment 3
[0093] formula:
[0094] 10 parts of Astragalus, 3 parts of Panax notoginseng and 4 parts of Danshen;
[0095] Preparation:
[0096] Mix the prescribed amount of astragalus, notoginseng and salvia miltiorrhiza, then add 10 times the weight volume of water to extract at 100°C for 3 hours, extract 4 times, combine the extracts, and concentrate under reduced pressure to 1.5±0.5g·mL based on the amount of medicinal materials -1 Concentration, add ethanol to 70-80%, let stand to precipitate, filter (or centrifuge), filtrate decompresses to recover ethanol, concentrate to 1.8±0.3g·mL based on the amount of medicinal materials -1 Concentration, to obtain the extract.
[0097] (2) Adsorb the extract with a macroporous resin, first elute impurities with water 10 times the weight of the macroporous resin, and then elute impurities with 10% ethanol at a volume concentration 5 times the weight of the macroporous resin;
[0098] (3) eluting with ethanol with a volume concentration of 8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com