Composition for amelioration/prevention of adverse side effect in steroid therapy
A composition and steroid technology, applied in the new use of branched-chain amino acids, the composition for inhibiting the expression of muscle atrophy-related genes, the combined use of the composition and steroid drugs, to prevent bedridden, shorten the hospitalization period and treat period, the effect of preventing recession
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
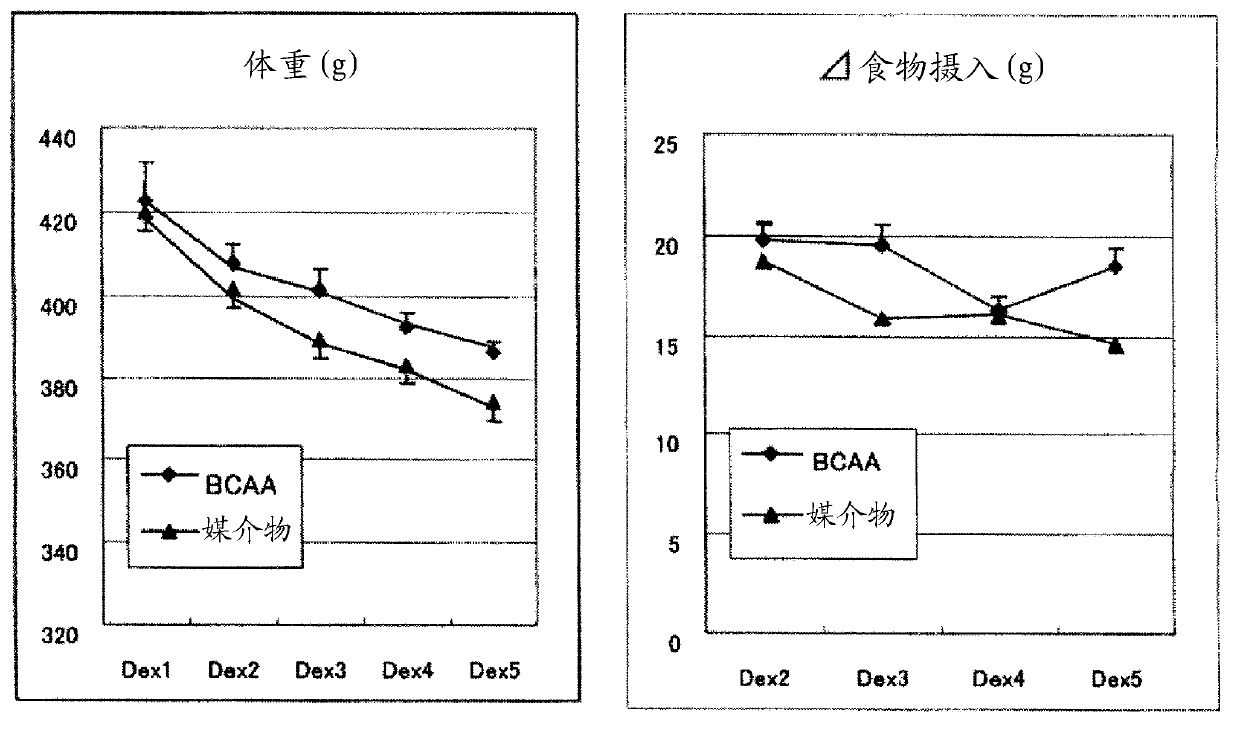
[0144] (Example 1) Muscular atrophy preventive effect of BCAA in dexamethasone-administered rats
[0145] Dexamethasone (600 μg / kg) was administered intraperitoneally to rats (SD; 10-11 weeks old) for 5 consecutive days. The isoleucine, leucine and valine (weight ratio 1:2:1.2) blend (BCAA) administration group was simultaneously orally administered with 0.75 g / kg of the above BCAA for 5 consecutive days. The vehicle group was sequentially administered orally with distilled water in the same manner.
[0146] On day 5, rats were necropsied and analyzed for functional assessment of muscle strength, blood glucose levels and plasma insulin levels using food intake, body weight profile, muscle weight, grip strength measurement devices.
[0147] As a control group, normal rats reared in pairs were used.
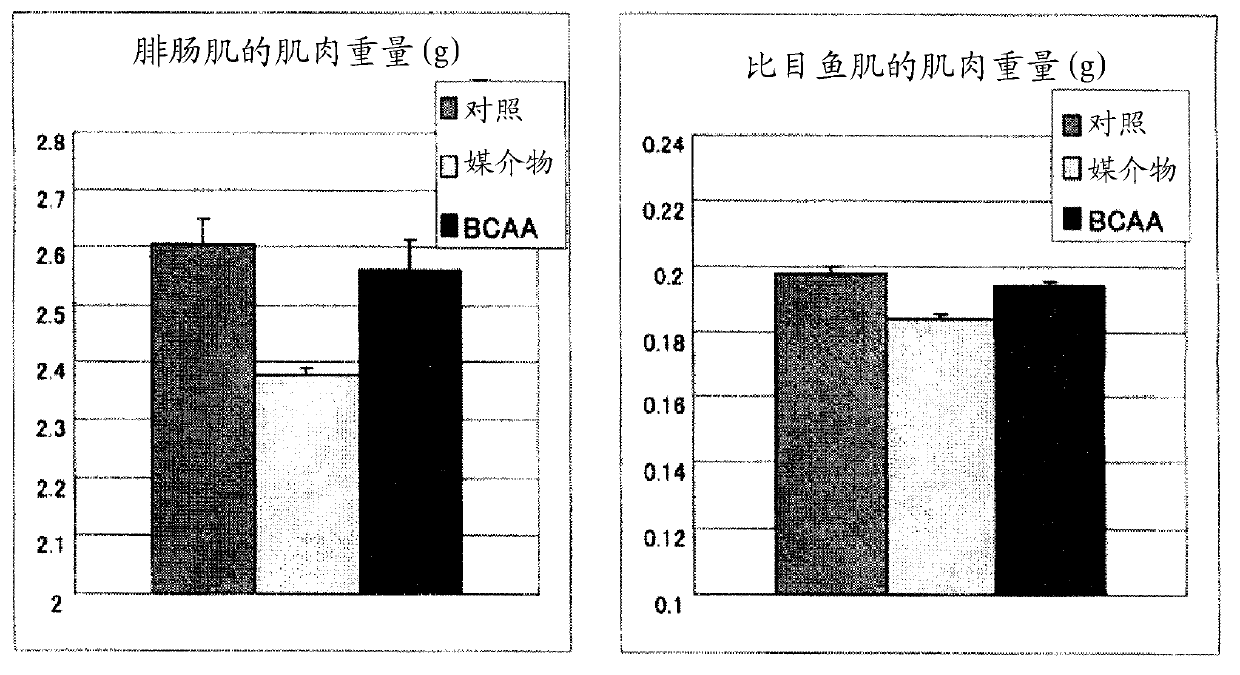
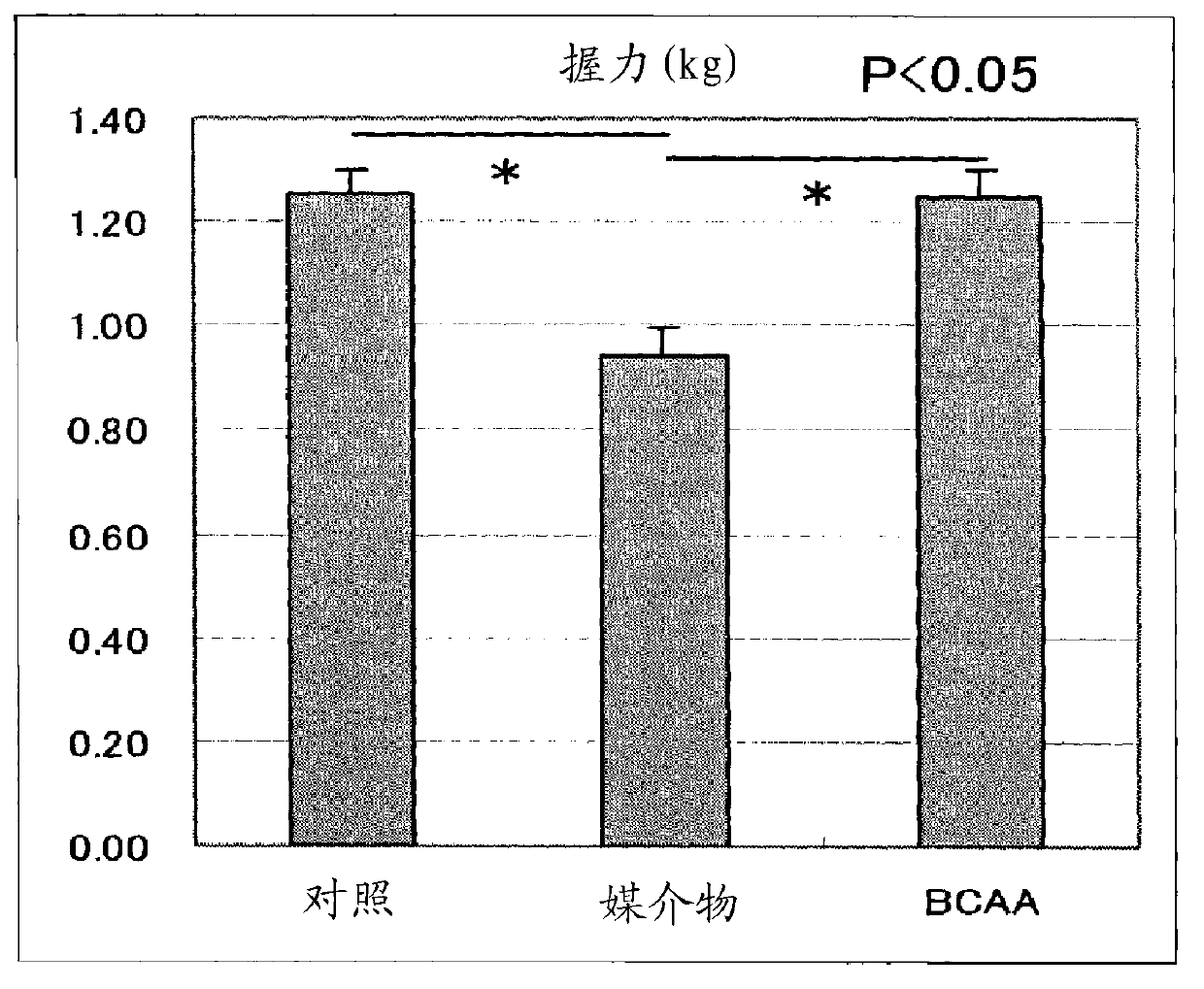
[0148] By comparison with the vehicle group, the BCAA administration group showed inhibition of body weight loss and reduction in food intake ( figure 1 ). The decrease in musc...
Embodiment 2
[0149] (Example 2) Muscle atrophy therapeutic effect of BCAA in rats administered with dexamethasone
[0150] Dexamethasone (600 μg / kg) was administered intraperitoneally to rats (SD; 10-11 weeks old) for 5 consecutive days to induce muscle atrophy. On the 6th and 7th days from the end of the dexamethasone administration, BCAA (0.75 g / kg) was orally administered, and the therapeutic effect on muscle atrophy was examined. The vehicle group was sequentially administered orally with distilled water in the same manner.
[0151] On days 6 and 7, rats were necropsied and analyzed for muscle weight. R1 and R2 are days 1 and 2, respectively, of the recovery period (recovery; R), and correspond to days 6 and 7 from the end of dexamethasone administration.
[0152] By comparison with the vehicle group, the BCAA administration group showed early recovery of muscle weight ( Figure 6 ).
Embodiment 3
[0153] (Example 3) Osteoporosis preventive effect of BCAA in dexamethasone-administered rats
[0154] Dexamethasone (600 μg / kg) was administered intraperitoneally to rats (SD; 10-11 weeks old) for 1.5 consecutive months. The isoleucine, leucine and valine (weight ratio 9:7:6) blend (BCAA) administration group was simultaneously orally administered with 0.75 g / kg of the above BCAA for 1.5 consecutive months. The vehicle group was sequentially administered orally with distilled water in the same manner.
[0155] At 1.5 months, rats were necropsied and analyzed for plasma ALP levels. It is known that plasma ALP shows a high value when bone metabolic turnover is high.
[0156] By comparison with the vehicle group, the BCAA administration group showed low plasma ALP values ( Figure 7 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com