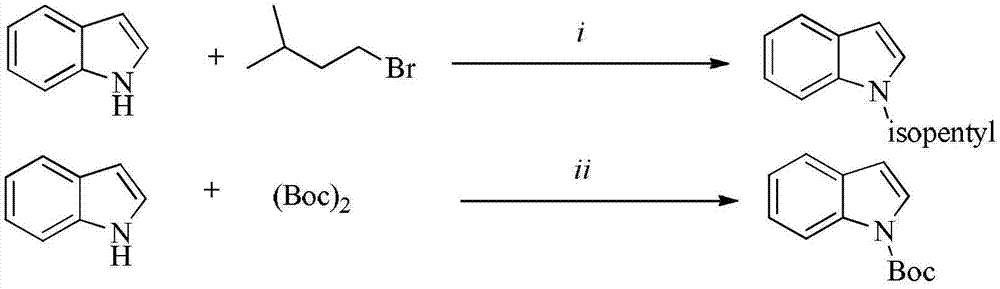
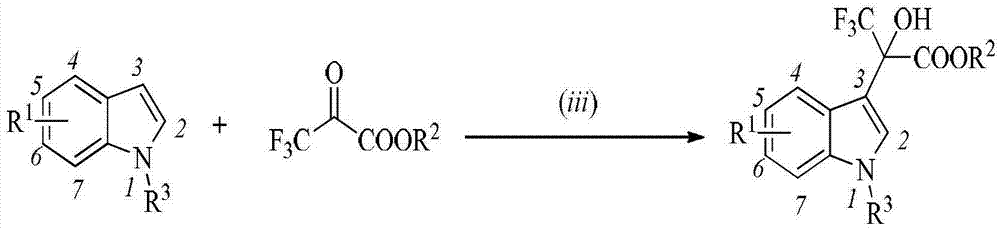Indole compound and application thereof as HIV-1 reverse transcriptase inhibitor
A technology of compounds and indoles, applied in the field of medicine, can solve the problems of complex structure, difficult synthesis, and not particularly good activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of ethyl 2-(5-nitro-1H-indol-3-yl)-2-hydroxy-3,3,3-trifluoropropionate (HX1)
[0037] Take 0.2mmol of 5-nitroindole and 0.2mmol of trifluoropyruvate into a single-necked round-bottomed flask containing a magnet, add 5mL of dichloromethane to dissolve, stir well, add 0.2mmol of AlCl 3 The Friedel-Crafts reaction was catalyzed, and the reaction was monitored by TLC. After the raw materials were fully reacted, the pure solid compound HX1 was separated by column chromatography, and the product was a yellow solid with a yield of 88%. 1 H NMR (400MHz, Acetone-d 6 )δ11.09(s,1H),8.85(d,J=1.7Hz,1H),7.96(dd,J=9.0,2.2Hz,1H),7.74(s,1H),7.53(d,J=9.0 Hz,1H),4.33–4.23(m,2H),1.20(t,J=7.1Hz,3H). 13 C NMR (101MHz, Acetone-d 6) δ 168.69, 142.80, 140.78, 129.76, 129.60, 126.41, 125.72, 119.27, 118.07, 113.13, 112.07, 64.07, 14.20.
Embodiment 2
[0038] Example 2: Preparation of ethyl 2-(5-fluoro-1H-indol-3-yl)-2-hydroxy-3,3,3-trifluoropropionate (HX2)
[0039] The preparation method was as in Example 1, and the product was a white solid with a yield of 90%. 1 H NMR (400MHz, CDCl 3 )δ8.38(s,1H),7.57(d,J=10.3Hz,1H),7.42(s,1H),7.20(s,1H),6.95(t,J=9.0Hz,1H),4.40( dq,J=20.9Hz,3H),1.33(t,J=7.1Hz,3H). 13 C NMR (101MHz, CDCl 3 )δ 169.19, 159.28, 132.90, 126.16, 125.57, 125.47, 124.93, 122.08, 112.14, 112.04, 111.38, 111.12, 108.67, 108.62, 106.40, 106.15, 64.42, 13.86.
Embodiment 3
[0040] Example 3: Preparation of ethyl 2-(5-chloro-1H-indol-3-yl)-2-hydroxy-3,3,3-trifluoropropionate (HX3)
[0041] The preparation method was as in Example 1, and the product was a white solid with a yield of 87%. 1 H NMR (400MHz, CDCl 3 )δ8.34(s,1H),8.00(s,1H),7.35(s,1H),7.18(d,J=10.3Hz,1H),7.11(d,J=8.6Hz,1H),4.40– 4.27(m,2H),1.28(t,J=7.1Hz,3H). 13 C NMR (101MHz, CDCl 3 ) δ 169.09, 135.03, 126.78, 125.70, 125.63, 124.82, 123.90, 121.97, 113.91, 112.81, 108.28, 64.51, 13.89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com