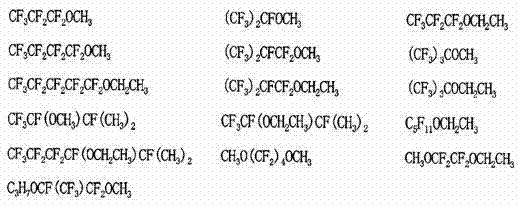
Fluorine-containing copolymer
一种共聚物、共聚的技术,应用在染色有机硅化合物处理、含有效成分的医用配制品、染色高分子有机化合物处理等方向,能够解决耐久性影响、使用环境条件影响、处理基材表面取向性降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
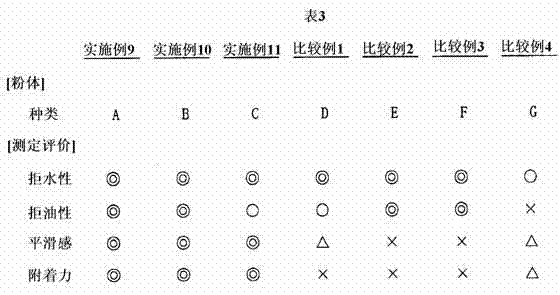
Examples
Embodiment
[0085] Next, the present invention will be described through examples.
Synthetic example 1
[0087] (1) Into a 1200ml autoclave equipped with a mixer and a thermometer
[0088] CF 3 (CF 2 ) 3 (CH 2 CF 2 )(CF 2 CF 2 )I (99.8GC%)
[0089] 603g (1.17 moles) and 7g of di-tert-butyl peroxide, and the autoclave was degassed with a vacuum pump. After heating the internal temperature to 80° C., ethylene was continuously introduced to adjust the internal pressure to 0.5 MPa. When the internal pressure decreased to 0.2 MPa, ethylene was introduced again so as to be 0.5 MPa, and the above operation was repeated. While maintaining the internal temperature at 80 to 115°C, 49 g (1.7 mol) of ethylene was introduced over about 3 hours. The content was recovered under the condition that the internal temperature was below 50°C to obtain 635g (98.9% yield) of
[0090] CF 3 (CF 2 ) 3 (CH 2 CF 2 )(CF 2 CF 2 )(CH 2 CH 2 )I (98.3GC%).
[0091] (2) In a three-necked flask with a capacity of 200 ml equipped with a condenser and a thermometer, add the
[0092] CF 3 (CF 2...
Synthetic example 2
[0102] (1) Used in the same manner as (1) in Synthesis Example 1
[0103] CF 3 (CF 2 ) 3 (CH 2 CF 2 )(CF 2 CF 2 ) 2 I (99.9GC%)
[0104] 529g (0.86 mol) and 5g of di-tert-butyl peroxide were introduced into the reaction of 34g (1.2 mol) of ethylene. As a result, 550g (yield 99.4%) of
[0105] CF 3 (CF 2 ) 3 (CH 2 CF 2 )(CF 2 CF 2 ) 2 (CH 2 CH 2 )I (99.1GC%).
[0106] (2) In a three-necked flask with a capacity of 200 ml equipped with a condenser and a thermometer, add the obtained in the above (4).
[0107] CF 3 (CF 2 ) 3 (CH 2 CF 2 )(CF 2 CF 2 ) 2 (CH 2 CH 2 )I (99.1GC%)
[0108] 150 g (0.24 mol) and 105 g (1.78 mol) of N-methylformamide were stirred at 150° C. for 5 hours. After completion of the reaction, the reaction mixture was washed with 40 ml of water, and the lower layer (132.3 g) was mixed with 135 g of a 15% by weight p-toluenesulfonic acid aqueous solution, and stirred at 80° C. for 7 hours. After the reaction mixture was left to st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com