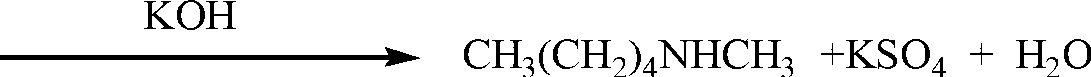Preparation method of N-methyl-pentylamine
A technology of methyl amylamine and n-amylamine, which is applied in the field of preparation of N-methyl amylamine, can solve the problems of long process route, influence on product promotion and application, low yield and the like, and achieves stable process and high yield. High, simple route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
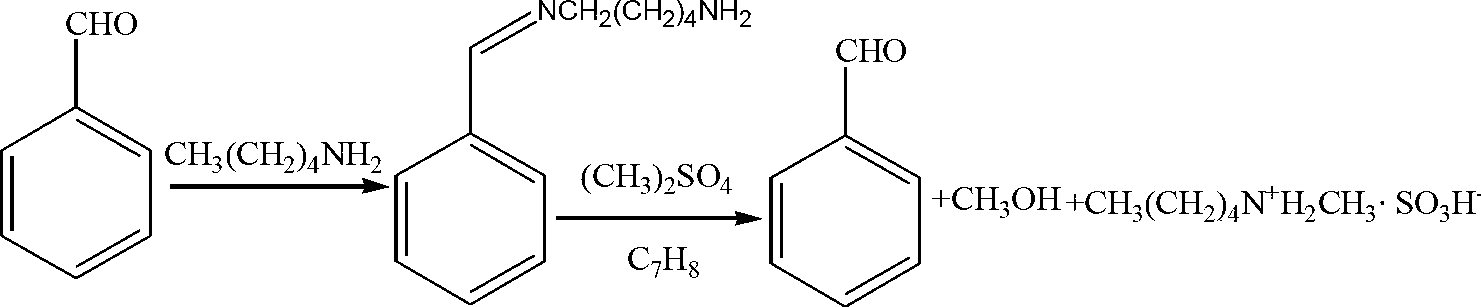
[0013] Add 100mL of anhydrous toluene, 57.8mL (0.5mol) of n-pentylamine and 66mL (0.65mol) of benzaldehyde into the reactor, heat and reflux for 30~40min; evaporate the solvent, cool the residue to 0~5℃, and keep the temperature condition Add 66.5mL (0.7mol) of dimethyl sulfate in anhydrous toluene (200mL), then heat to reflux for 20~25min, remove toluene and benzaldehyde under reduced pressure; cool the remaining solution to -5~0℃, add solid KOH, adjust pH=9.0, separate the amine layer, and distill under reduced pressure to obtain N-methylpentylamine (colorless transparent liquid, b.p.116-117°C, product purity 98.3%, yield 72.3%).
Embodiment 2
[0015] Add 120mL of anhydrous toluene, 57.8mL (0.5mol) of n-pentylamine and 71mL (0.9mol) of benzaldehyde into the reactor, heat and reflux for 30~40min; evaporate the solvent, cool the residue to 0~5℃, and keep the temperature condition Add 66.5mL (0.7mol) of dimethyl sulfate in anhydrous toluene (210mL), heat and reflux for 20~25min, remove toluene and benzaldehyde under reduced pressure; cool the remaining liquid to -5~0℃, add solid KOH, adjust pH=8.0, separate the amine layer, and distill under reduced pressure to obtain N-methylpentylamine (colorless transparent liquid, b.p.116-117°C, product purity 98.7%, yield 71.9%).
Embodiment 3
[0017] Add 150mL of anhydrous toluene, 57.8mL (0.5mol) of n-pentylamine and 76mL (0.75mol) of benzaldehyde into the reactor, heat and reflux for 30~40min; evaporate the solvent, cool the residue to 0~5℃, and keep the temperature condition Add 71mL (0.75mol) of dimethyl sulfate in anhydrous toluene (150mL), heat and reflux for 20~25min, remove toluene and benzaldehyde under reduced pressure; cool the remaining solution to -5~0℃, add solid KOH , adjust the pH=9.0, separate the amine layer, and distill under reduced pressure to obtain N-methylpentylamine (colorless transparent liquid, b.p.116-117°C, product purity 98.8%, yield 72.6%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com