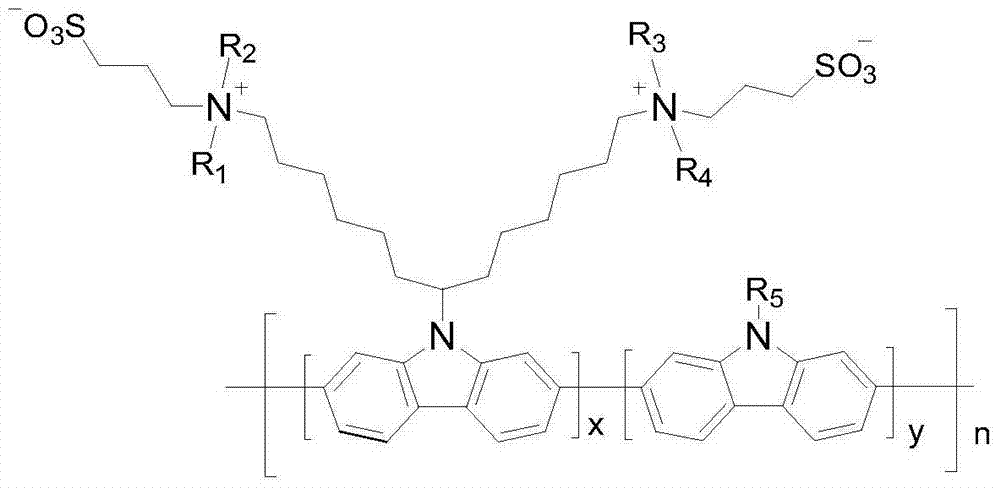
Conjugate polyelectrolyte photoelectric material based on poly-carbazole and application of conjugate polyelectrolyte photoelectric material based on poly-carbazole
A technology of conjugated polyelectrolyte and optoelectronic materials, applied in the direction of organic chemistry, etc., can solve the problems of low conductivity, reduce the life and stability of OLED devices, PVK hole injection/transport performance is not very good, etc., to achieve high conductivity efficiency, and the effect of avoiding adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
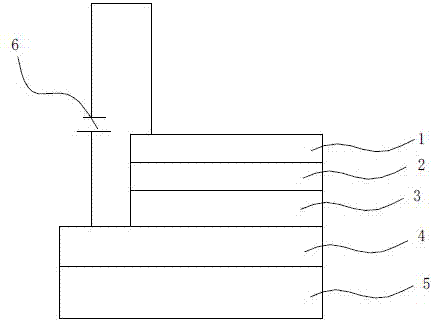
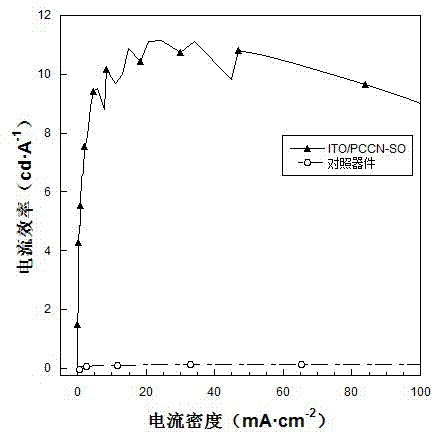
Image
Examples
Embodiment 1
[0023] Preparation of 4,4-dibromo-2-nitrobiphenyl (1)
[0024] Dissolve 4,4-dibromobiphenyl (20g, 64mmol) in glacial acetic acid (300mL), heat and stir at 110°C, add concentrated nitric acid (70%, 132mL) dropwise to glacial acetic acid, heat for 6h until solid Slowly dissolve, then cool to room temperature. The reaction solution was filtered, the solid was washed with water, and then recrystallized with ethanol to obtain a yellow solid (17.6g, yield77%). 1 H-NMR (300MHz, CDCl 3 ): (ppm) 8.32 (d, 2H), 8.29 (d, 2H), 7.77 (dd, 2H).
Embodiment 2
[0026] Preparation of 2,7-dibromocarbazole (2)
[0027] Triethyl phosphate (60 mL) added with compound 1 (16.5 g, 46.1 mmol) was heated to reflux for 18 h under nitrogen protection. The excess solvent was distilled off from the reaction liquid under reduced pressure, and the residue was dissolved with a small amount of dichloromethane, then precipitated with petroleum ether to obtain the initial product, and then a white solid (9.28 g, yield 62%) was obtained by silica gel / petroleum ether column chromatography. 1 H-NMR (300MHz, CDCl 3 ): δ (ppm) 8.20 (br, 1H, NH); 7.92 (d, 2H); 7.56 (d, 2H); 7.35 (dd, 2H).
Embodiment 3
[0029] Preparation of p-methylphenyl-1-bromo-6-hexyl ether (3)
[0030] Add p-cresol (28g), 1,6-dibromohexane (220mL), potassium carbonate (60g), tetrabutylammonium bromide (3.8g) and acetone (150mL) into a 500mL flask, heat and stir to reflux 48h. The reaction was cooled to room temperature, filtered, and the filtrate was spun with a circulating water pump until the mass was no longer reduced, then distilled under reduced pressure with an oil pump, and the fraction collected at 210°C to 220°C was a colorless oily product (53.5g, yield76.1%). 1 H-NMR (300MHz, CDCl 3 ): δ (ppm) 7.10 (d, 2H); 6.82 (d, 2H); 3.96 (t, 2H); 3.45 (t, 2H); 2.32 (s, 3H); 1.8 (br, 8H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com