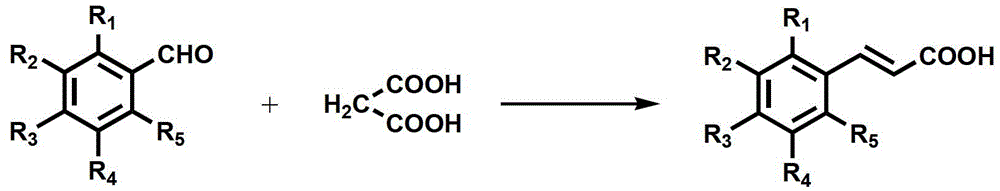Substituted benzhydryl piperazine derivative and preparation method and application thereof
A technology of benzhydrylpiperazine and derivatives is applied in the field of substituted benzhydrylpiperazine derivatives and their preparation, and the preparation of brain neuroprotective agents, which can solve the problem of poor oral bioavailability and difficulty in permeating blood. Brain barrier, neurobehavioral toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (E)-3-(4-ethoxyphenyl)acrylic acid
[0045] Add 4-hydroxybenzaldehyde (5.09g, 0.0417mol) and water (25mL) into the reaction flask, heat up to 55°C, add tetrabutylammonium bromide (1.07g, 0.0033mol), wash with 30%wtNaOH solution Adjust the pH to make it alkaline. After the 4-hydroxybenzaldehyde is completely dissolved (the solution is orange-red), continue to heat up to boiling, reflux, and then add diethyl sulfate (6.68g, 5.66mL, 0.0433mol) dropwise, and finish dropping in 20 minutes , add 30%wt NaOH solution to ensure that the reaction solution is alkaline, after reflux for 20 hours, stop the reaction, separate the solution, dissolve the organic layer with 20mL toluene, wash with 5%wt NaOH solution (10mL*3), wash with water 3 times, dried over anhydrous magnesium sulfate, and evaporated to dryness to obtain 4.6 g of the product white solid 4-ethoxybenzaldehyde, yield 70.8%.
[0046] Add 4-ethoxybenzaldehyde (8.5g, 0.0566mol), malonic acid (8.8g, 0.0849mol), pyridine (...
Embodiment 2
[0048] (E)-3-(2-ethoxyphenyl)acrylic acid
[0049] The preparation method is the same as in Example 1, 2-hydroxybenzaldehyde (10.18g, 8.8mL, 0.0834mol), diethyl sulfate (13.36g, 11.3mL, 0.0866mol), tetrabutylammonium bromide (2.14g, 0.0066mol ), reflux reaction for 5.5 hours, to obtain 9 g of yellow liquid, yield 69.2%.
[0050] 2-Ethoxybenzaldehyde (15g, 0.0999mol), malonic acid (15.6g, 0.15mol), pyridine (68mL), hexahydropyridine (1.8mL), heated to 95°C to obtain 10.8g of white solid, Yield 56.3%.
Embodiment 3
[0052] (E)-3-(4-methylphenyl)acrylic acid
[0053] The preparation method is the same as in Example 1, 4-methylbenzaldehyde (12.0g, 0.1mol), malonic acid (15.6g, 0.15mol), pyridine (68mL), hexahydropyridine (1.8mL), heated to 95°C for reaction , 12.3 g of white solid was obtained, yield 75.9%, mp 195.1-196°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com