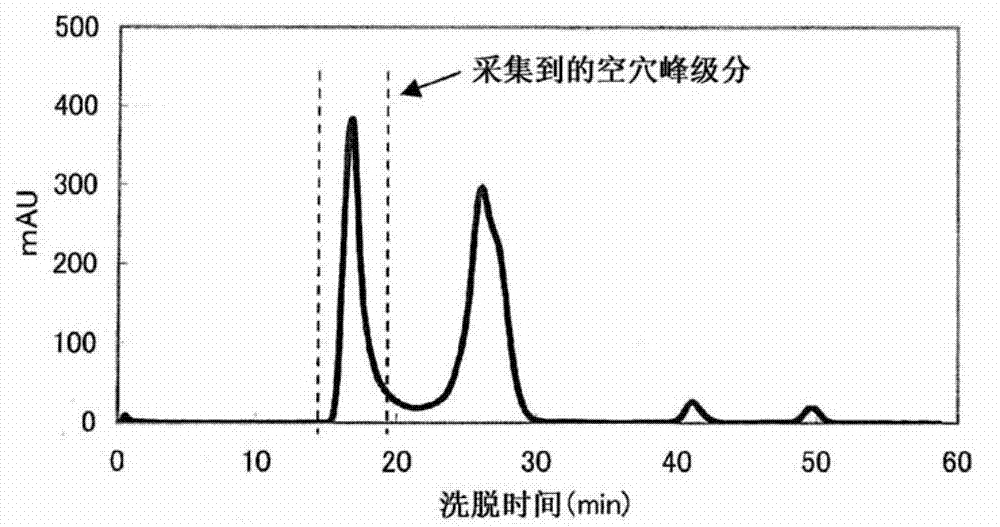
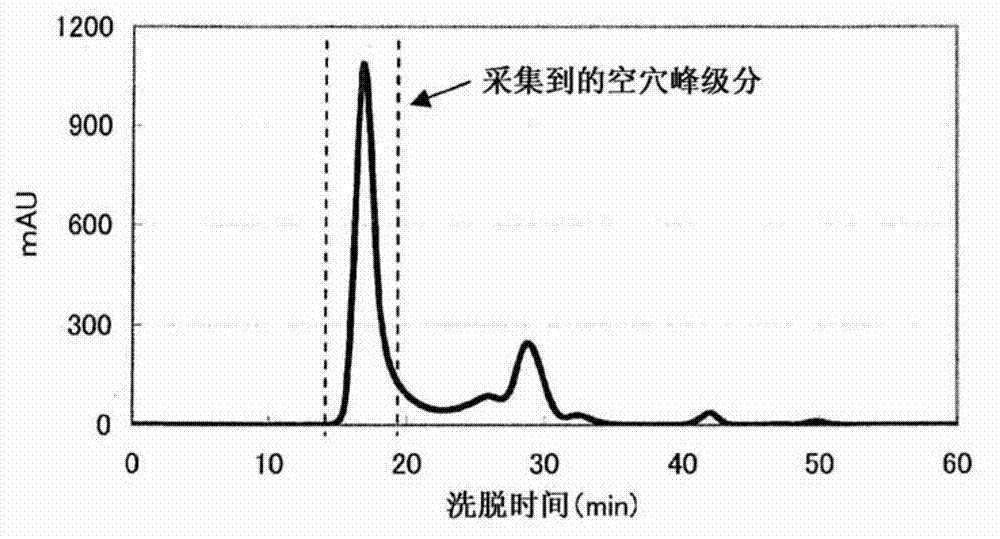
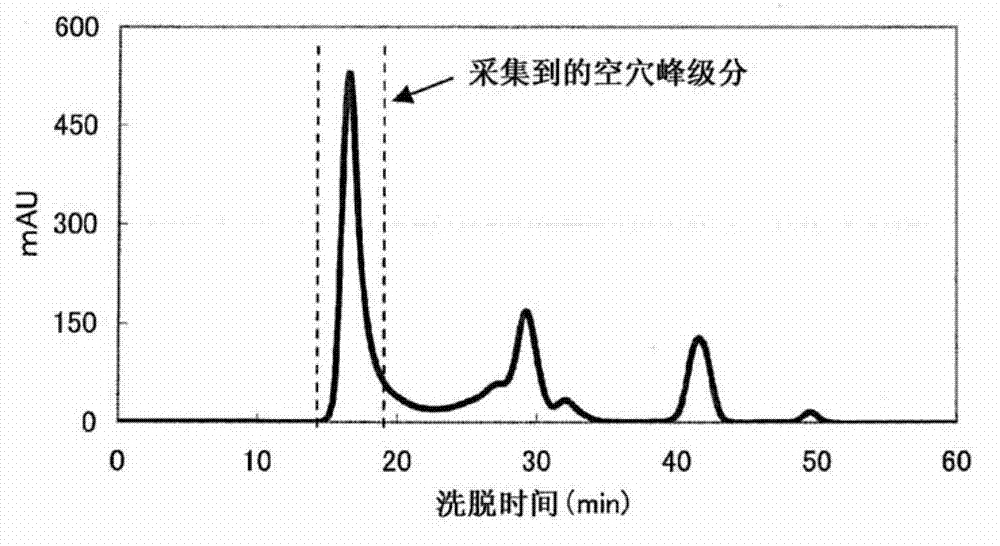
Complex of labeled probe and water-soluble carrier
一种水溶性载体、复合物的技术,应用在标记化探针-水溶性载体复合物领域,能够解决难以得到高灵敏度且稳定的复合物等问题,达到再现性良好、灵敏度高、保持经时稳定性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of IgG with Thiols by Reductive Treatment
[0061] Anti-HCV core antigen mouse monoclonal IgG antibodies c11-9 and c11-14 were dissolved in 0.1 M phosphate buffer (pH 7.2) to prepare 5 mg / mL solutions, respectively. 30 µL of 0.2M 2-mercaptoethanol (manufactured by Wako Pure Chemical Industries, Ltd.) was added to 300 µL of these IgG solutions, and the reaction was carried out at 37° C. for 1.5 hours. After the reaction, gel filtration purification was performed using PD-10 (manufactured by GE Healthcare) to obtain IgG having a thiol group obtained by reducing a disulfide bond present in the antibody. As a result of quantifying the number of each thiol group, it was confirmed that 8.0 thiol groups existed per IgG molecule.
Embodiment 2
[0063] Preparation of Fab' with thiol groups by reduction treatment
[0064] Anti-HCV core antigen mouse monoclonal IgG antibodies c11-9 and c11-14 were dissolved in 0.1 M sodium acetate buffer (pH 4.5) to prepare 10 mg / mL. Next, 0.2 mg of pepsin was added to 1 mL of these IgG solutions, and stirred at 37° C. for 6 hours to digest the IgG with pepsin. After digestion with pepsin, it was neutralized with 2NNaOH, and further purified with a Superdex 200 column (manufactured by GE Healthcare) to obtain F(ab') 2 .
[0065] To each 3.0mg / mL F(ab') 2 130 μL of 0.2 M 2-mercaptoethanol (manufactured by Wako Pure Chemical Industries, Ltd.) was added to 1300 μL, and the reaction was carried out at 37° C. for 1.5 hours. After the reaction, gel filtration purification was performed using PD-10 (manufactured by GE Healthcare) to obtain Fab' having a thiol group obtained by reducing a disulfide bond present in the body. The number of each thiol group was quantified, and as a result, 3...
Embodiment 3
[0067] Introduction of maleimide groups into streptavidin
[0068] 20 mg of streptavidin (manufactured by MP Bio) was dissolved in 2 mL of 0.1 M phosphate buffer (pH 7.2), and a maleimide reagent dissolved at 6 mg / mL was added to dimethylformamide Sulfo-KMUS (manufactured by Dojin Chemical Co., Ltd.) 133 μL. After reacting at 30° C. for 1 hour, it was purified by gel filtration using PD-10 (manufactured by GE Healthcare) to obtain streptavidin having a maleimide group introduced therein. As a result of quantifying the number of maleimide groups, it was confirmed that 3.7 maleimide groups were present per one molecule of streptavidin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com