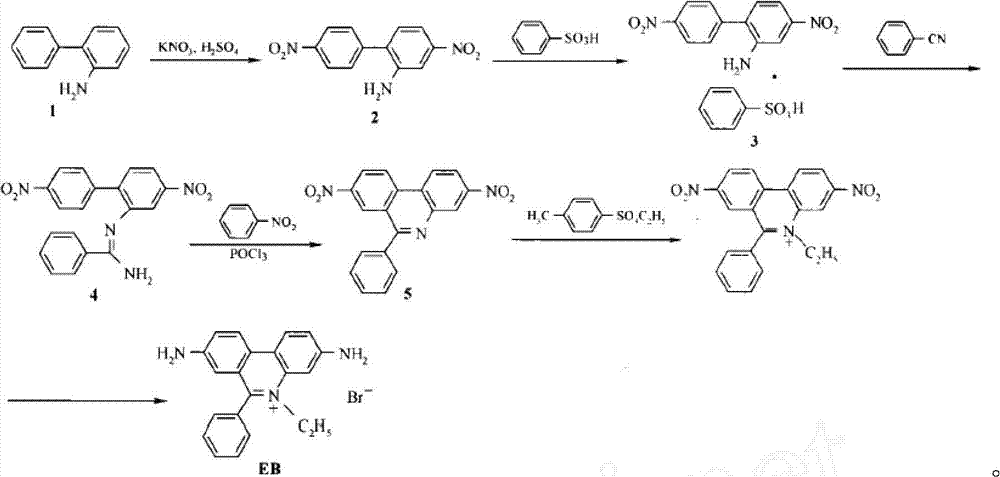
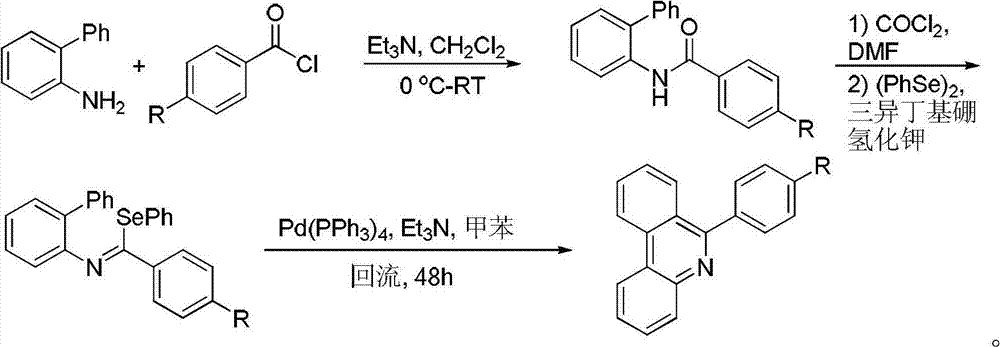
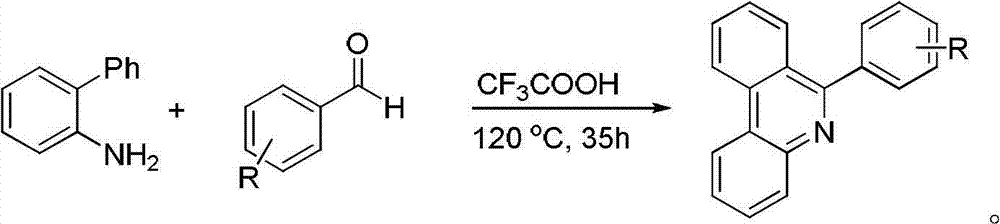
Preparation method for phenanthridine derivative
A derivative, phenanthridine technology, applied in the field of organic synthesis, can solve the problems of practical application difficulties, high reaction temperature, long time, etc., and achieve the effects of strong designability, simple post-processing, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0041] Add catalyst, oxidizing agent, acid, 2-aminobiphenyl compound (II), olefin compound (III) and organic solvent 2ml in the round bottom flask of 10ml according to the raw material ratio of Table 1, mix and stir evenly, according to the reaction of Table 2 The conditions were to stir the reaction in the air for a specified time. After the reaction was completed, it was filtered, mixed with silica gel, and purified by column chromatography (eluent was petroleum ether and ethyl acetate, volume ratio=10:1) to obtain the corresponding phenanthridine derivative Thing (I), reaction process is shown in the following formula:
[0042]
[0043] Table 1
[0044]
[0045] Table 2
[0046]
[0047] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, Ph is phenyl, TFEtOH is trifluoroethanol, EtOH is ethanol, Ph-p-Me is p-methylphenyl, Ph-p-OMe is P-methoxyphenyl, Ph-p-Cl is p-chlorophenyl; R in formula (I-7) 3 For the addition of oxycarbonyl ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com