Murraya paniculata leaf and application of mono compound in preparation of sedative and hypnotic drugs
A technology of the leaves and extracts of Gurgifolium japonica, which is applied in the field of application of the extracts and monomeric compounds of Gulis japonica in the preparation of sedative and hypnotic drugs. Slow and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
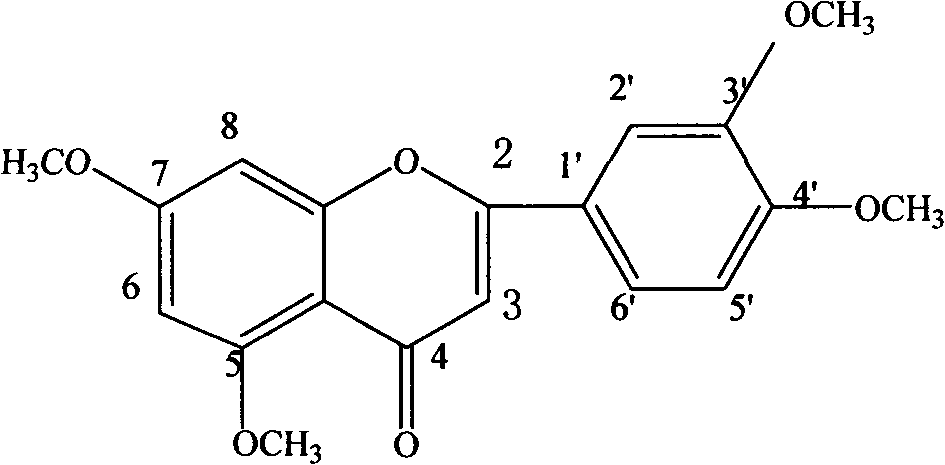
[0051] Example 1 Extraction and separation of 5,7,3',4',5'-pentamethoxyflavone and 5-hydroxy-6,7,8,3',4'-pentamethoxyflavone from the leaves of Gurifera , 5,7,3',4'-Tetramethoxyflavone
[0052] (1) Grind 1Kg of dried Gilija leaves, add water to decoct and extract 3 times, the amount of water added is 15 liters, 12 liters and 10 liters respectively, the decoction time is 2 hours, 1.5 hours and 1 hour respectively, and the three extracts are combined . The extract was adsorbed by an AB-8 macroporous adsorption resin column, washed with water, desorbed with 85% ethanol, and the ethanol was recovered to obtain 112 grams of jiulixiang leaf extract.
[0053] (2) Preliminary separation of flavonoids
[0054] Al 2 o 3 Column (2kg), eluting with ethyl acetate. The eluate was collected separately, detected by TLC, and the same components were combined to obtain J 1 、J 2 , and J 3 three parts.
[0055] (3) Separation and purification of monomeric compounds
[0056] Part J1 was ...
Embodiment 2
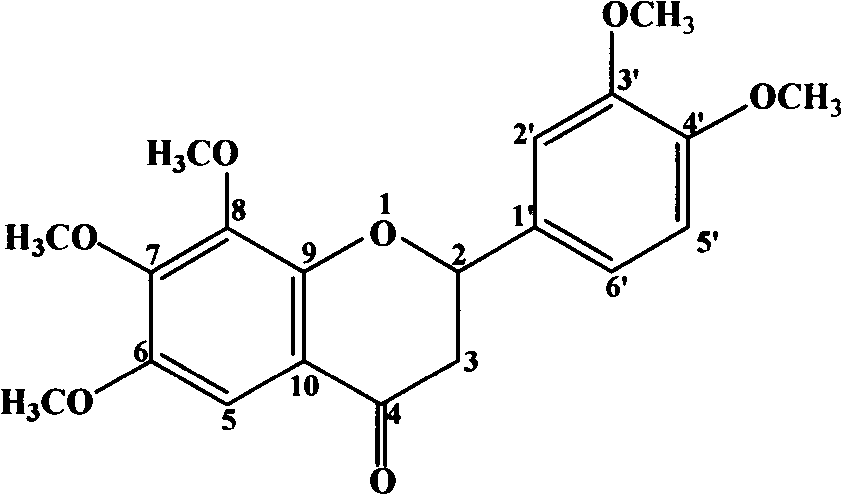
[0066] Example 2 Extraction and separation of 6, 7, 8, 3', 4'-pentamethoxy dihydroflavone, 2'-hydroxy-3,4,5,4',6'-pentamethoxyl Chichalcone
[0067] (1) Extraction
[0068] Take 5kg of dried coriander leaves and pulverize, and extract three times with 85% ethanol solution under reflux. The ethanol used for each time is 30L, 30L and 30L respectively, and the extraction time is 3h, 2h and 1h respectively. The three extracts were combined, concentrated to a certain extent, diluted with water to pass the AB-8 macroporous adsorption resin, eluted with 95% ethanol solution, and the solvent was recovered to obtain 500 g of extract.
[0069] (2) Separation and purification
[0070] The obtained 500g extract was subjected to silica gel column chromatography with petroleum ether:ethyl acetate-2:1 as the eluent, and six fractions A-F were obtained. Yellow crystals were precipitated in part B, filtered and recrystallized with ethyl acetate to obtain 2'-hydroxy-3,4,5,4',6'-pentamethoxyc...
Embodiment 3 9
[0076] Example 3 Preparation of Gulina officinalis extract A
[0077] Take 10 kilograms of dried coriander leaves, add water and reflux for extraction 5 times, the amount of water added is 14, 12, 10, 8, and 6 times, respectively, and the reflux time is 120, 90, 60, 60, and 45 minutes, respectively, filter, and combine the extracts , the solvent was recovered under reduced pressure, and dried to obtain the extract A of Fructus Fructus. Detected by HPLC, it contains 5,7,3',4',5'-pentamethoxyflavone, 6,7,8,3',4'-pentamethoxydihydroflavone, 5-hydroxy-6 , 7,8,3',4'-pentamethoxyflavone, 2'-hydroxy-3,4,5,4',6'-pentamethoxychalcone and 5,7,3',4 '-Tetramethoxyflavone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com