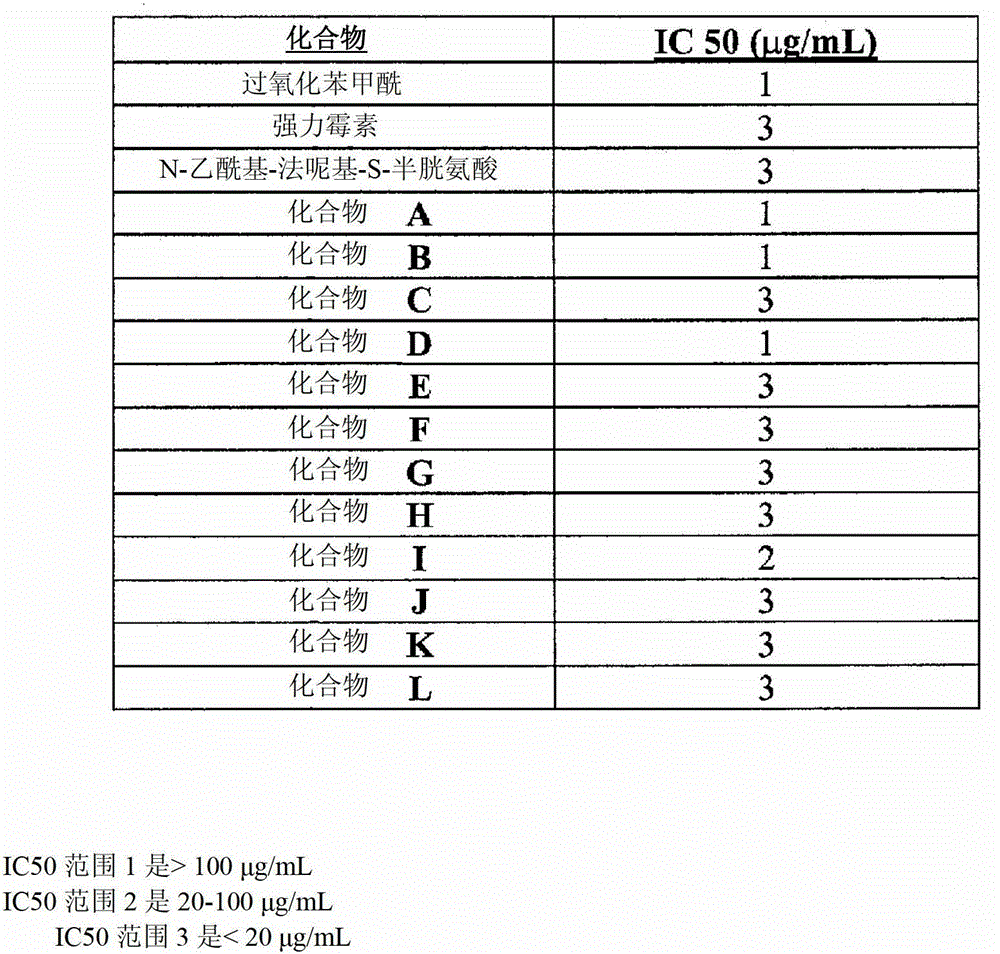
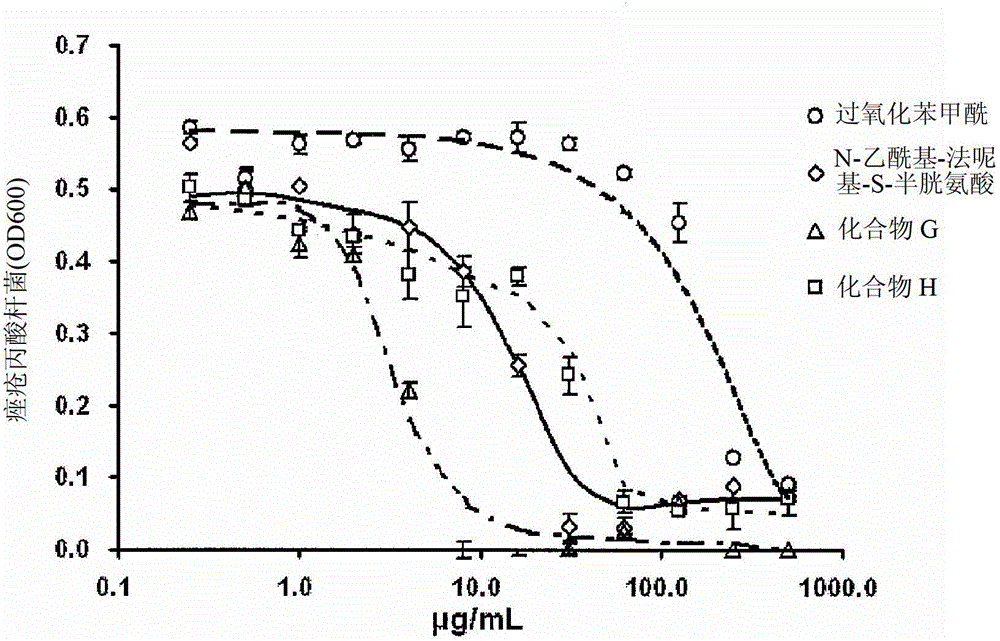
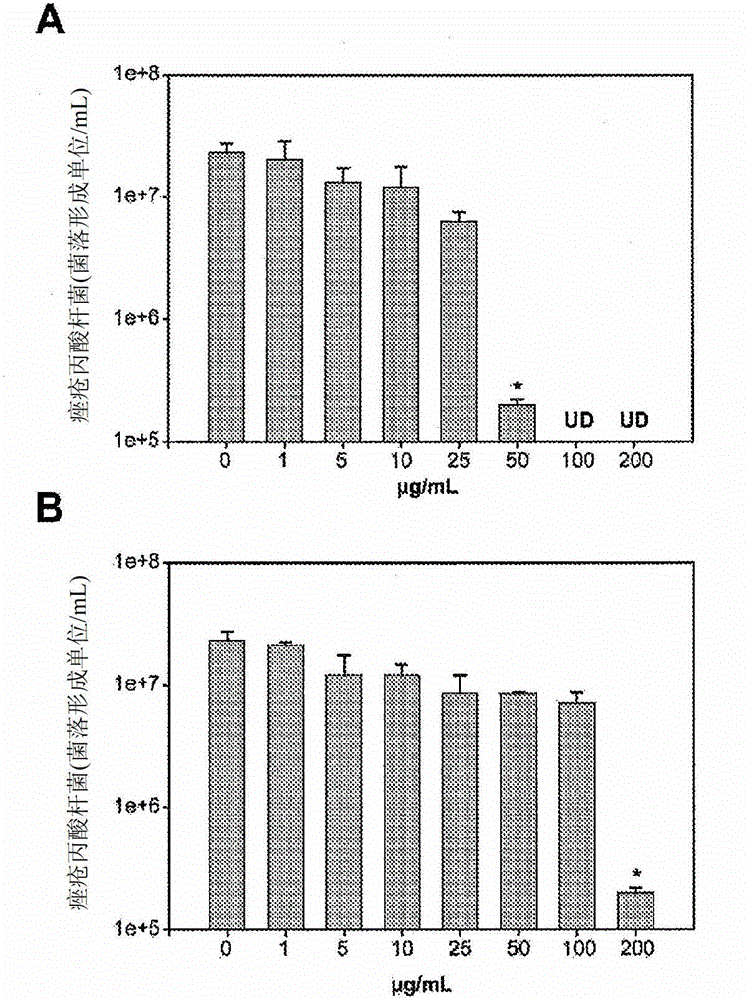
Use of an antibacterial agent for the treatment of an epithelial-related condition
一种用途、病况的技术,应用在抗细菌剂用于治疗上皮相关病况的用途领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0354]
[0355] (4-((R)-1-carboxy-2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienylthio)ethylamino)- Synthesis of 4-oxobutanoic acid) (Compound B): To a solution of S-trans,trans-farnesyl-L-cysteine (500 mg, 1.54 mmol) in THF was added the first copies of K 2 CO 3 (2 mmol) and the resulting solution was cooled to 5°C while stirring vigorously. To this stirred solution was added succinic anhydride (308 mg, 3.1 mmol) dropwise while utilizing another portion of K 2 CO 3 (4 mmol) to maintain the pH at 9.0-10.0. The mixture was stirred at room temperature for 2 h, HPLC analysis showed the reaction was complete. The pH of the reaction mixture was then adjusted to 2.0 by adding 2N HCl solution. The acidic solution was extracted 3 times with 10 mL of ethyl acetate. The combined organic extracts were washed with water, brine and washed with Na 2 SO 4 Drying and removal of solvent on a rotary evaporator afforded crude compound B, which was further purified by preparative HP...
example 2
[0357]
[0358] ((E)-4-((R)-1-carboxy-2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienylthio)ethyl Synthesis of S-trans,trans-farnesyl-L-cysteine (500mg, 1.54mmol) in THF and the first part of K 2 CO 3 (3 mmol) was cooled to 5°C. To this stirred solution was added maleic anhydride (302mg, 3.07mmol) portionwise while utilizing another portion of K 2 CO 3 (3 mmol) to maintain the pH at 9.0-10.0. The mixture was stirred at room temperature for 3 h, HPLC analysis showed the reaction was complete. The pH of the reaction mixture was then adjusted to 2.0 by adding 2N HCl solution. The acidic solution was extracted 3 times with 15 mL of ethyl acetate. The combined organic extracts were washed with water, brine and washed with Na 2 SO 4 Drying and then concentration afforded crude Compound A, which was further purified by preparative HPLC (552 mg, 85%) to yield Compound A. 1 H-NMR (500MHz, CD 3 OD): δ1.50 (bs, 6H), 1.57 (s, 3H), 1.59 (s, 3H), 1.85-2.10 (m, 8H), 2.68 (dd, ...
example 3
[0360]
[0361] ((R)-2-((S)-2-Acetamido-3-hydroxypropionylamino)-3-((2E,6E)-3,7,11-trimethyldodeca-2,6, 10-trienylthio)propionic acid) and ((R)-2-((R)-2-acetylamino-3-hydroxypropionylamino)-3-((2E,6E)-3,7, 11-Trimethyldodeca-2,6,10-trienylthio)propionic acid)) Synthesis of a mixture (compound D): In a 100 mL round bottom flask, N-acetyl-L-serine ( 1.0mmol), coupling reagent (520mgPBOP or 380mgHATU, 1mmol) and N, N-diisopropyl-ethylamine (650mg, 5mmol) were mixed in CH 2 Cl 2 (10mL). The reaction mixture was stirred at room temperature for 30 minutes. S-trans,trans-farnesyl-L-cysteine (325 mg, 1 mmol) was added to the reaction mixture and stirred overnight at room temperature. CH removal by rotary evaporation 2 Cl 2 . The resulting residue was dissolved in ethyl acetate (50 mL). use NH 4 Cl saturated solution (50mL) washed the organic solution, washed with Na 2 SO 4 Dried and concentrated to obtain a crude mixture. The crude mixture was purified by preparative ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com