Application of bisbenzylisoquinoline compound or pharmaceutically acceptable salt thereof in preparing medicine or healthcare product for improving sleep
A bisbenzylisoquinoline, sleep-improving technology, applied to medical preparations containing active ingredients, pharmaceutical formulas, drug combinations, etc., can solve problems such as no research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
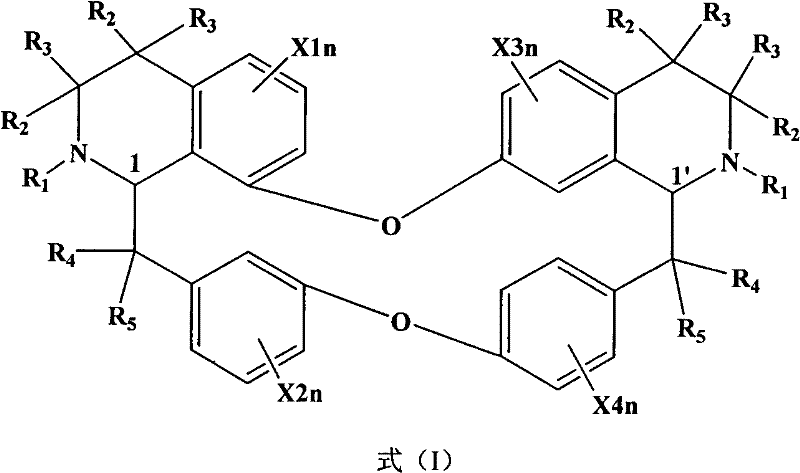
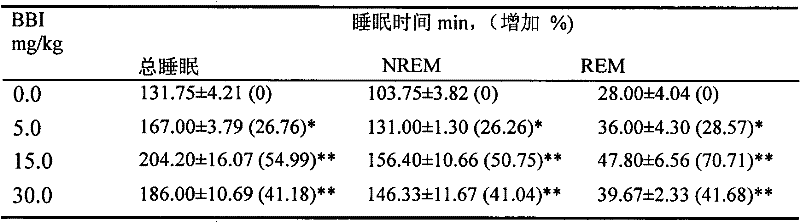
[0012] Experiment 1. Effects of bisbenzylisoquinoline (BBI) derivatives represented by formula (I) on total sleep time and sleep phase (REM and NREM sleep) of mice after administration for 1, 3, and 7 days respectively.
[0013] The mice were taken, and electroencephalogram (EEG) and electromyography (EMG) electrodes were installed after anesthesia. One week after the operation, they were divided into random groups. The control group was given vehicle by intragastric administration, and the other groups were administered intragastrically with BBI derivatives at doses of 1, 15, and 30 mg / kg for 1 day, 3 days and 7 days respectively. Immediately after the last administration, the EEG and EMG were recorded for 8 hours. The EEG and EMG were analyzed with SleepSign for Animal 2.0 software, and the results are shown in Table 1. The experimental results showed that intragastric administration of the derivatives for 1 day (Table 1), 3 days (Table 2), and 7 days (Table 3) could dose-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com