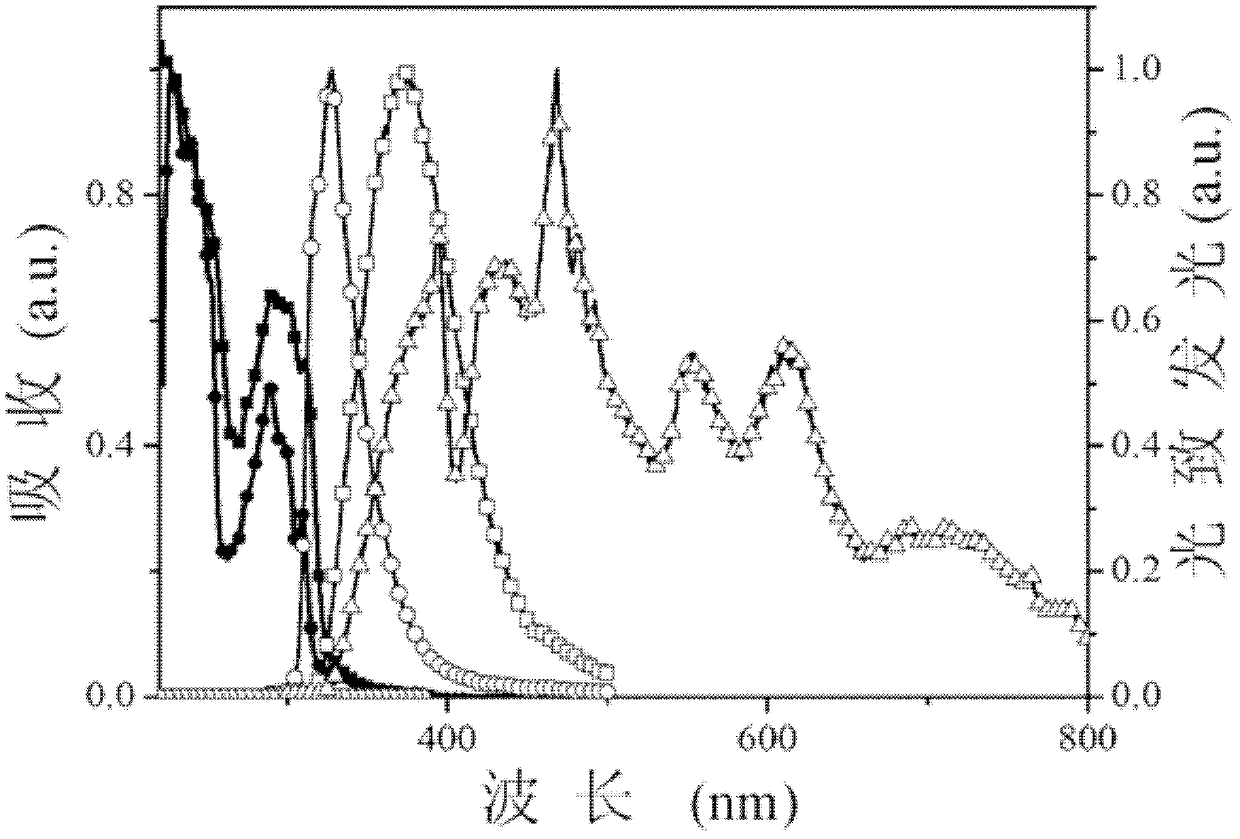
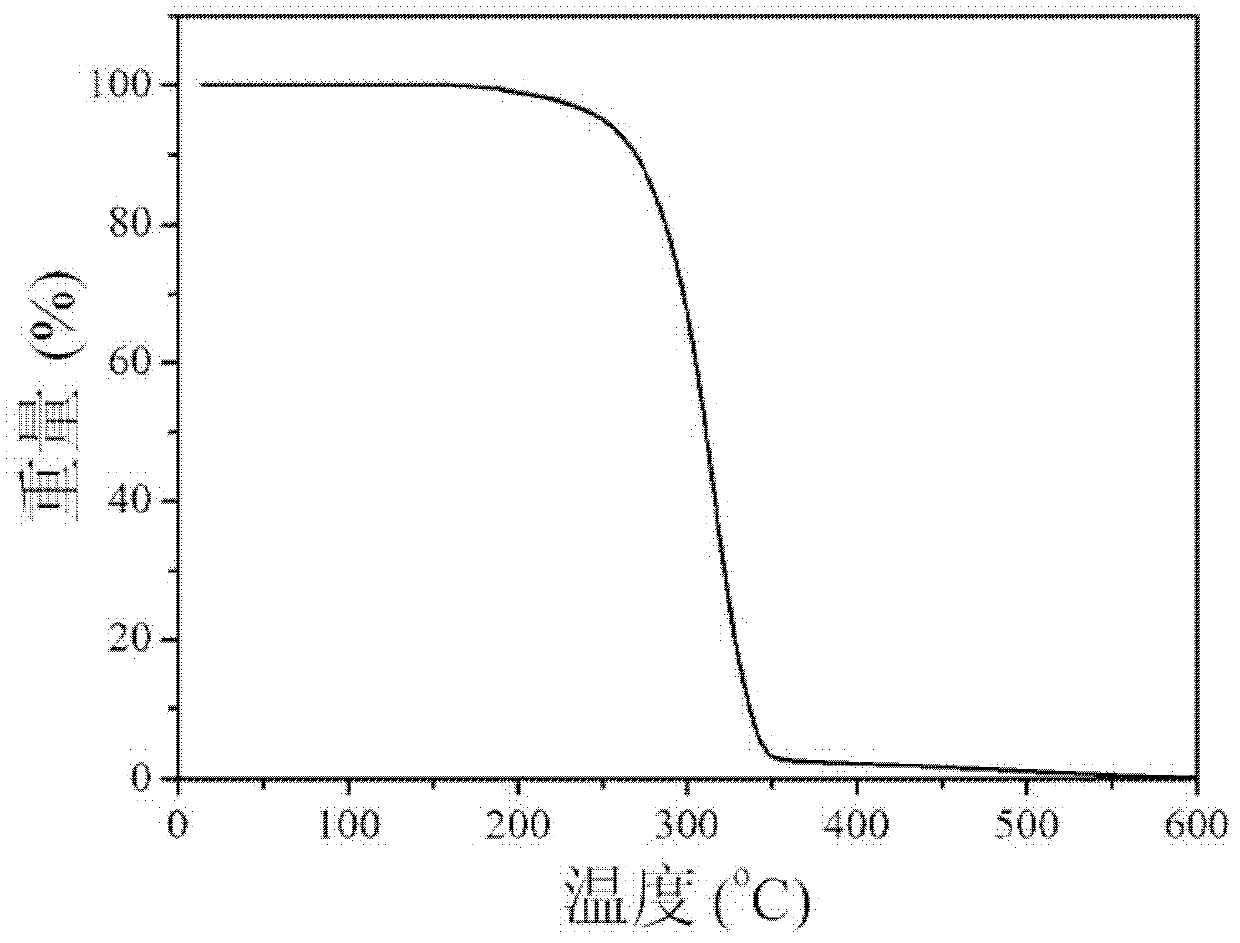
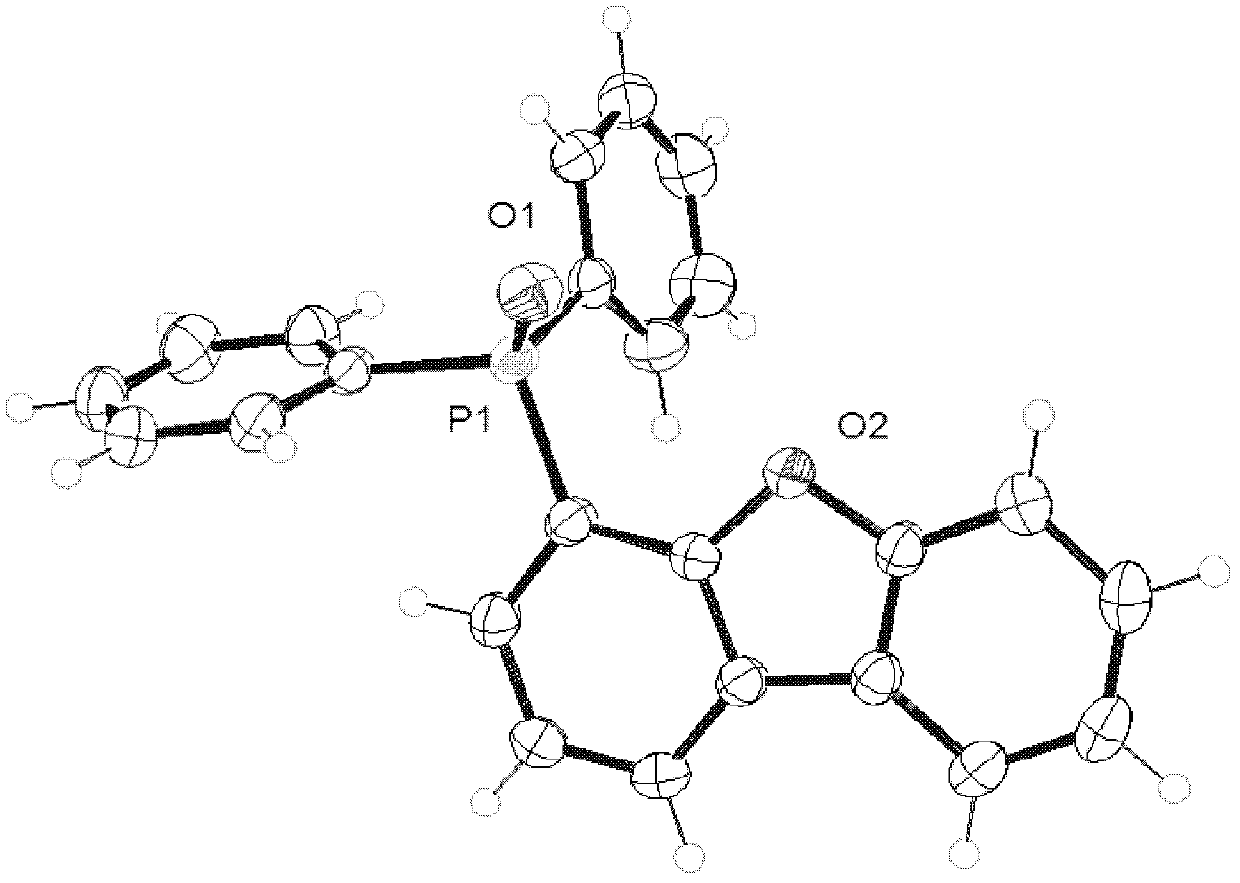
Dibenzofuranyl aromatic phosporic-oxygenic compound and preparation method and application thereof
A technology of dibenzofuranyl and diphenylphosphine oxide, which is applied in the field of dibenzofuranyl aromatic phosphine oxides, can solve the problems of concentration quenching, high driving voltage of phosphorescent devices, low luminous efficiency and brightness, etc. Excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific embodiment 1: This embodiment is a dibenzofuryl aromatic phosphine oxide compound, which is based on dibenzofuran, and the diphenylphosphine group is at the 4-position or 4,6-position of dibenzofuran Substituted obtained, its structural general formula is as follows:
[0027] Wherein, X is H or a diphenylphosphine group.
specific Embodiment approach 2
[0028] Embodiment 2: This embodiment is different from Embodiment 1 in that X is H. Other parameters are the same as in the first embodiment.
[0029] The dibenzofuryl aromatic phosphine oxide compound of this embodiment is obtained by using dibenzofuran as a parent, and the diphenylphosphine group is substituted at the 4-position of dibenzofuran, and its structural formula is as follows:
[0030] Abbreviated as o-DBFPPO.
specific Embodiment approach 3
[0031] Embodiment 3: This embodiment is different from Embodiment 1 in that X is a diphenylphosphine group. Other parameters are the same as in the first embodiment.
[0032] The dibenzofuryl aromatic phosphine oxide compound of this embodiment is obtained by using dibenzofuran as the parent, and the diphenylphosphine group is substituted at the 4 and 6 positions of dibenzofuran, and its structural formula is as follows:
[0033] Abbreviated as o-DBFDPO.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com