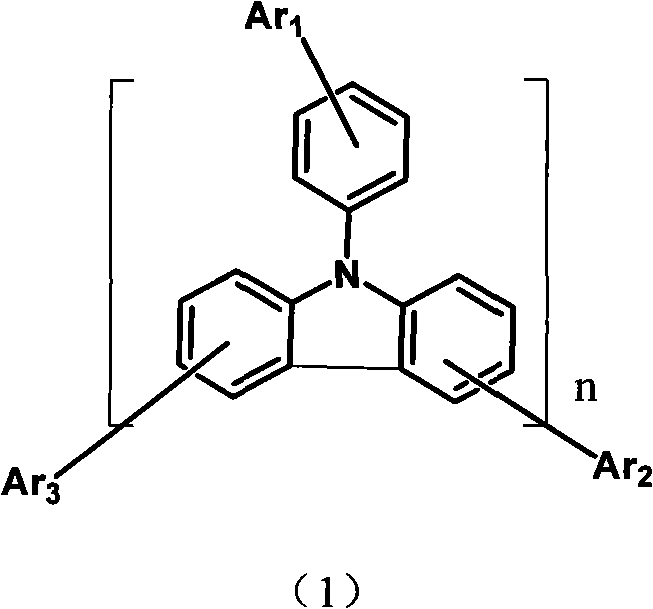
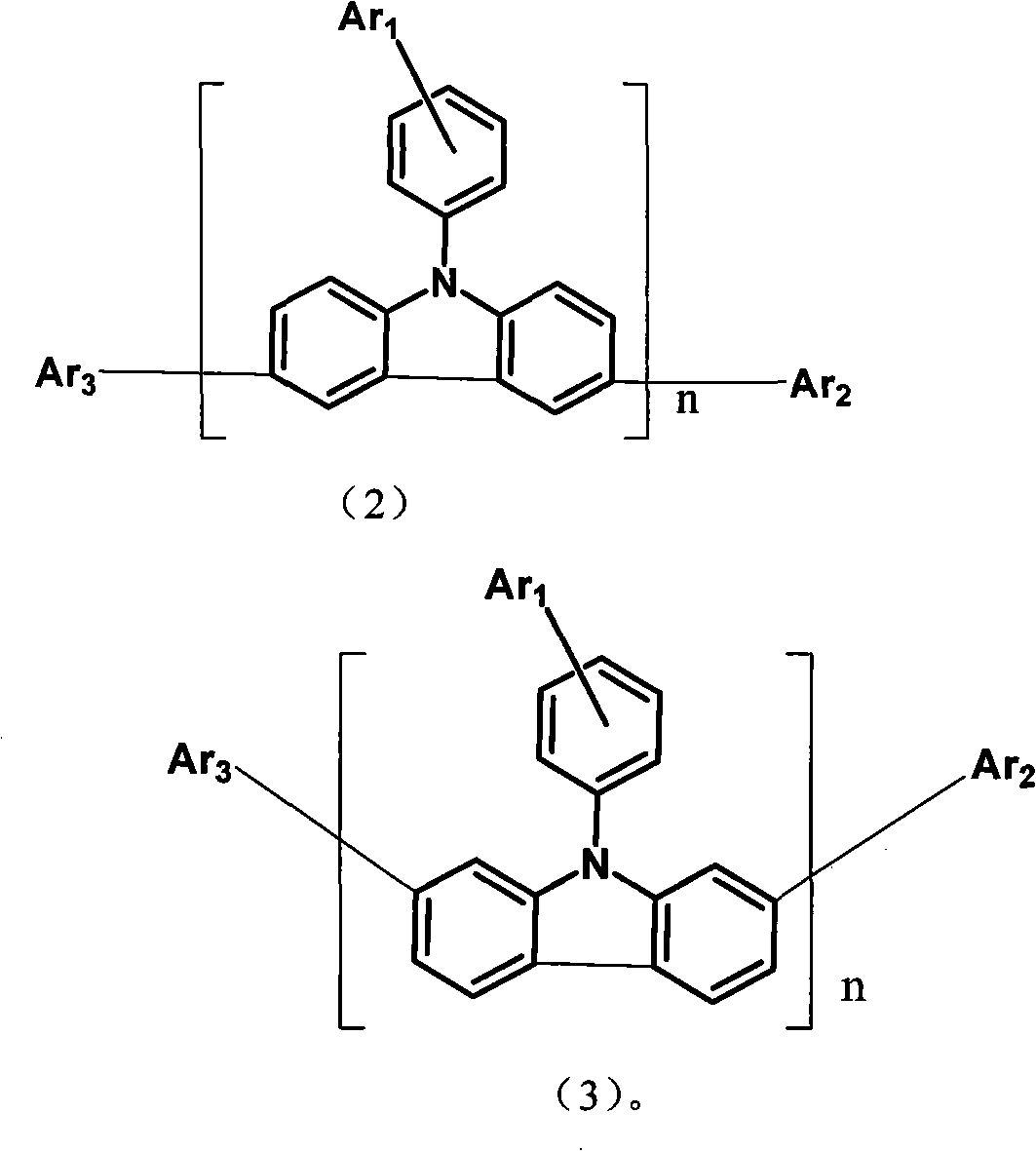
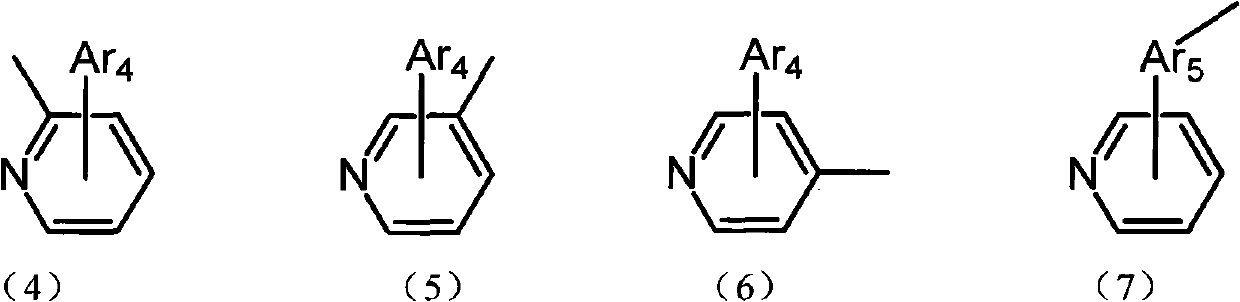
Aryl pyridine ring-contained carbazole compounds and application thereof
A technology of compound and pyridine ring, which is applied in the field of new compounds, can solve the problems of large differences in luminous efficiency and achieve high electron and hole mobility and good thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The synthesis of embodiment 1 intermediate
[0065] (1) Synthesis of M101
[0066]
[0067] 25.85g 2,6-dibromopyridine, 13.20g phenylboronic acid and 0.55g Pd(PPh 3 ) 4 Dissolve in a mixture of 330mL toluene and 220mL ethanol, dissolve 24.2g potassium carbonate in 110mL water and add to the above reaction solution, stir and react at 70°C for 1.5h, then add 2.75g phenylboronic acid, react for another 0.5h, monitor by TLC end point of the reaction. After the reaction was completed, the organic phase was separated, washed three times with water and washed with anhydrous Na 2 SO 4 Carry out column chromatography after drying, eluent is sherwood oil: methylene chloride=20: 1 (V 1 / V 2 ), to obtain 15.60 g of white solid. MS (m / e): 234, yield 61.0%.
[0068] (2) Synthesis of M102
[0069]
[0070] 33.84g of 2-iodo-5-bromopyridine, 15.95g of phenylboronic acid and 0.55g of Pd(PPh 3 ) 4 Dissolve in a mixture of 330mL toluene and 220mL ethanol, dissolve 24.2g po...
Embodiment 2
[0138] The synthesis of embodiment 2 target product 1-1
[0139]
[0140] 47.9g M116, 55.3g 3-pyridineboronic acid and 1.15g Pd(PPh 3 ) 4 Dissolve in a mixture of 500mL toluene and 220mL ethanol, dissolve 24.2g potassium carbonate in 110mL water and add to the above reaction solution, stir and react at 70°C for 3h, and monitor the end point of the reaction by TLC. After the reaction was completed, the organic phase was separated, washed three times with water and washed with anhydrous Na 2 SO 4 Carry out column chromatography after drying, eluent is sherwood oil: methylene chloride=20: 1 (V 1 / V 2), to obtain 37.92g of white solid. MS (m / e): 474, yield 80.0%. Elemental analysis (C 33 h 22 N 4 ): theoretical value C: 83.52%, H: 4.67%, N: 11.81%; measured value C: 83.61%, H: 4.68%, N: 11.71%.
Embodiment 3
[0141] The synthesis of embodiment 3 target product 1-2
[0142] Using M116 and 2-pyridineboronic acid as raw materials, compound 1-2 was obtained through the same reaction as in Example 2. MS (m / e): 474, elemental analysis (C 33 h 22 N 4 ): theoretical value, C: 83.52%, H: 4.67%, N: 11.81%; measured value C: 83.62%, H: 4.69%, N: 11.69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com