Membrane type-1 matrix metalloprotein inhibitors and uses thereof
一种基质金属、膜型的技术,应用在蛋白酶抑制剂、肽/蛋白质成分、抗炎剂等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
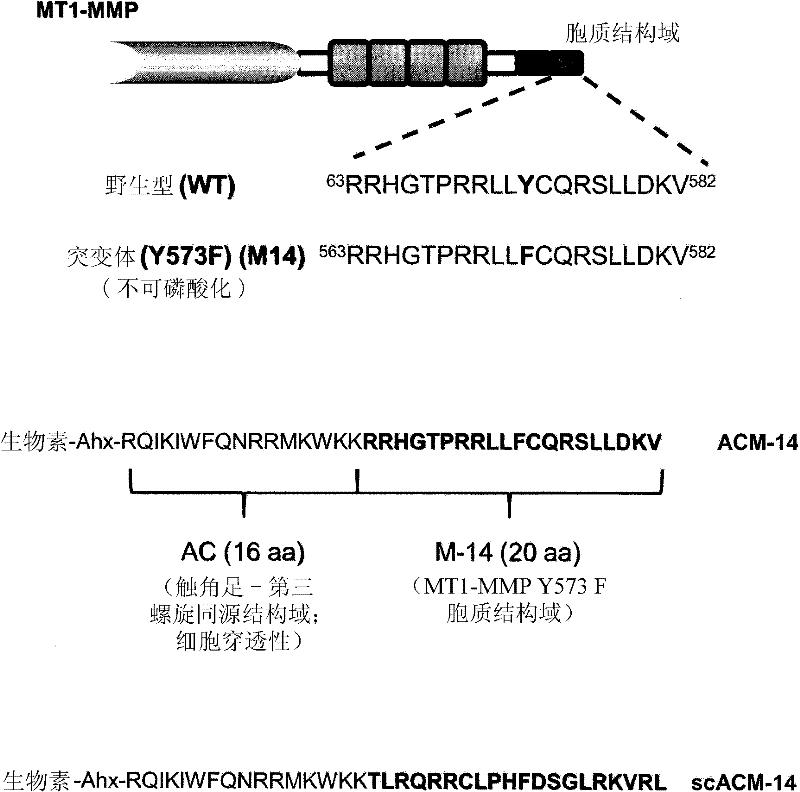
[0262] Preparation of MT1-MMP-derived peptides
[0263] In carrying out the experiments described in the Examples below, the following peptides were prepared. First, a peptide with the sequence of the cytoplasmic domain of MT1-MMP (RRHGTPRRLLLYCQRSLLDKV; SEQ ID NO: 117) and a non-phosphorylatable form of this peptide (RRHGTPRRLLFCQRSLLDKV; SEQ ID NO: 118) were prepared, wherein in combination with human MT1- The tyrosine at the position corresponding to amino acid 573 of the MMP sequence was replaced by phenylalanine. This non-phosphorylatable peptide was referred to as "M-14".
[0264] Additionally, a peptide with the M-14 sequence fused to the third helix of the cell-penetrating tentaclepedin homology domain (RQIKIWFQNRRMKWKK; SEQ ID NO: 119) was made. This fusion peptide is called ACM-14 and has the sequence: Biotin-Ahx-RQIKIWFQNRRMKWKK-RRHGTPRRLLFCQRSLLDKV (SEQ ID NO: 176). A version of the fusion peptide in which the cytoplasmic MT1-MMP domain sequence was scrambled wa...
Embodiment 2
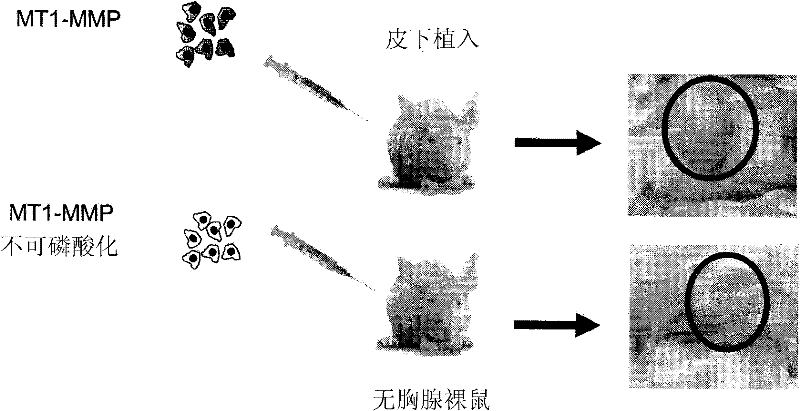
[0266] Expression of Y573F MT1-MMP inhibits tumor growth
[0267] HT1080 fibrosarcoma cells were stably transfected with WT MT1-MMP or Y573F MT1-MMP. HT1080 cells are very aggressive cancer cells that express elevated levels of MT1-MMP. These two groups of cells were transplanted subcutaneously into athymic nude mice, and tumor growth was monitored. Such as figure 2 As shown, cells expressing the cytoplasmic domain of MT1-MMP exhibited significant tumor growth, whereas no tumor growth was observed in mice receiving cells expressing the Y573F mutant.
Embodiment 3
[0269] ACM-14 and scACM-14 efficiently taken up by fibrosarcoma cells
[0270] To determine whether ACM-14 and scACM-14 were able to enter tumor cells, HT-1080 fibrosarcoma cells were incubated with each peptide (1 μM) for 1 hr, and peptide uptake was analyzed by immunofluorescence and confocal microscopy. Such as image 3 As shown, ACM-14 and its promiscuous form (scACM-14) were visualized in this cell, indicating efficient and rapid cellular uptake.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com