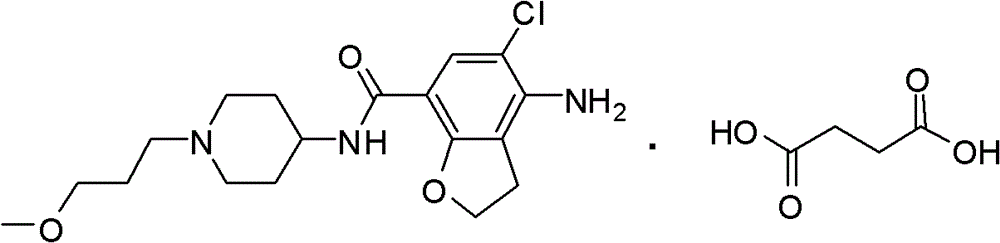
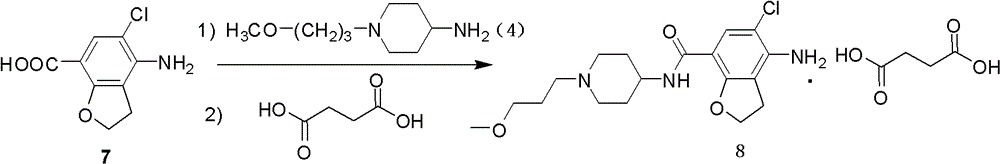
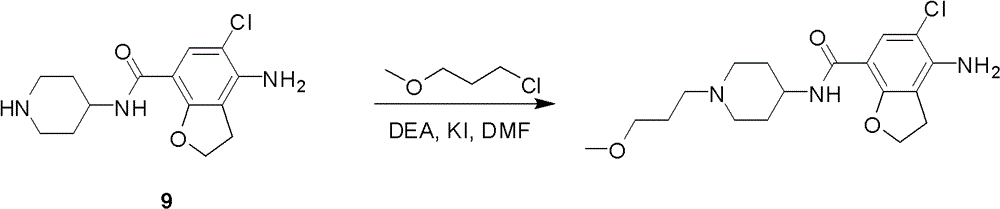
4-n-substituted-1-(3-methoxypropyl)-4-piperidinamine compounds and their preparation and application
A technology of methoxypropyl and piperidinamine, applied in the application field of synthesizing prucalopride succinate, achieving the effect of convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of 4-N-tert-butoxycarbonyl-1-(3-methoxypropyl)-4-piperidinamine (1-1)
[0041] Add 4-Boc-aminopiperidine (30.04g, 0.15mol), 1-bromo-3-methoxypropane (22.95g, 0.15mol), potassium carbonate (20.73g, 0.15mol) and acetonitrile in a 1000ml reaction flask (500ml), reflux for 5h, concentrate under reduced pressure, add water (200ml) and stir, filter, wash the filter cake with water (100ml), and dry to obtain off-white solid 4-N-tert-butoxycarbonyl-1-(3-methyl Oxypropyl)-4-piperidinamine (39.11 g, yield 95.85%), melting point 72.5-73.5°C.
[0042] MS (m / z): 273.22 ([M+H] + )
[0043] 1 H-NMR (400MHz, DCCl 3 )δ: 1.41 (9H, s), 1.59 (2H, m), 1.60-1.86 (4H, m), 2.43-2.53 (6H, m), 3.36 (3H, s), 3.42 (2H, t), 3.63 (1H, m).
Embodiment 2
[0045] Preparation of 1-(3-methoxypropyl)-4-piperidinamine (4)
[0046] 4-N-tert-butoxycarbonyl-1-(3-methoxypropyl)-4-piperidinamine (39.11g, 0.144mol), concentrated hydrochloric acid (250ml) and ethanol (500ml) were added to the reaction flask, room temperature After reacting for 3h, NaOH (20g) in ethanol (200ml) was added after concentration under reduced pressure. After concentration, dichloromethane (200ml) was added, filtered, and the filtrate was concentrated to give light yellow oil 4 (21.75g, yield 87.9%).
[0047] MS (m / z): 173.18 ([M+H]+)
Embodiment 3
[0049] Preparation of 4-N-benzyloxycarbonyl-1-(3-methoxypropyl)-4-piperidinamine (1-2)
[0050] 4-Cbz-aminopiperidine (23.43g, 0.1mol), 1-bromo-3-methoxypropane (15.30g, 0.1mol), potassium carbonate (8.30g, 0.1mol) and acetone (250ml) were added to 500ml In the reaction flask, reflux for 5h. Concentrate under reduced pressure, add water, stir, filter, and dry to give white solid 4-N-benzyloxycarbonyl-1-(3-methoxypropyl)-4-piperidinamine (24.97 g, 81.49%).
[0051] MS (m / z): 307.21 ([M+H]+)
[0052] 1H-NMR (400MHz, DCCl3) δ: 1.59 (2H, m), 1.62-1.85 (4H, m), 2.43-2.53 (6H, m), 3.36 (3H, s), 3.42 (2H, t), 3.63 (1H, m), 5.16 (2H, s), 7.30-7.51 (5H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com