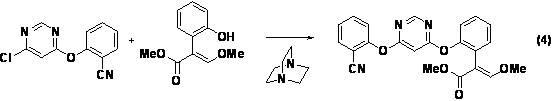Preparation method of azoxystrobin and its key intermediate
A technology of azoxystrobin and intermediates, which is applied in the field of fungicides synthesis, can solve the problems of long reaction time sequence and low reaction activity, and achieves the effects of short reaction time sequence, simple post-processing, and reduction of post-processing and purification steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
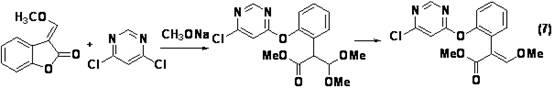
[0045] Example 1 Preparation of azoxystrobin key intermediate 2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3,3-dimethoxypropionic acid methyl ester
[0046] Under nitrogen protection, in a 250mL three-necked flask, methyl 2-(2-hydroxyphenyl)-3,3-dimethoxypropionate (compound II) (24.0g, 0.10mol), 4,6- Dichloropyrimidine (Compound IV) (16.3g, 0.11mol) was dissolved in 60mL N,N-dimethylformamide, and anhydrous potassium carbonate (20.7g, 0.15mol) was added, heated to 60°C for 3h, and then Add 2-cyanophenol (compound III) (11.9g, 0.10mol) and 1,4-diazabicyclo[2.2.2]octane (0.15g, 1mmol) into the reaction flask, keep warm at 60°C, 2.5 h, determined by high performance liquid chromatography, the test results showed that 2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3,3-dimethoxy was generated Methyl propionate (compound ).
[0047] The above reaction mixture was cooled and concentrated under reduced pressure (water bath temperature of 80°C), dissolved in toluene (...
Embodiment 2
[0050] Example 2 Synthesis of Azoxystrobin
[0051] step one:
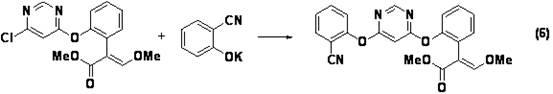
[0052] Under nitrogen protection, methyl 2-(2-hydroxyphenyl)-3,3-dimethoxypropionate and (E)-2-(2-hydroxyphenyl)-3-methoxyacrylate Dissolve 20.4g of the ester mixture (HPLC area peak: 93:7) in 40mL of N,N-dimethylformamide, add 13.8g of 4,6-dichloropyrimidine, 17.6g of anhydrous potassium carbonate, heat up to 60°C for 3h . Add 11.6g of 2-cyanophenol to the reaction flask and continue the reaction for 5h. The reaction mixture was cooled and concentrated under reduced pressure (using a water bath temperature of 80°C) to obtain a brown-red solid, which was dissolved in toluene (100 mL), washed with 50 mL of water and concentrated under reduced pressure (using a water bath temperature of 60°C) to obtain crude Product 30.5g. That is, methyl 2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3,3-dimethoxypropionate and (E)-2-{ Methyl 2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3-methoxyacrylate mixture (HPLC ...
Embodiment 3
[0057] Example 3: Methyl 2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3,3-dimethoxypropionate and (E)-2- Preparation of methyl {2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3-methoxyacrylate:
[0058] Methyl 2-[2-(6-chloropyrimidin-4-ylmethoxy)phenyl]-3,3-dimethoxypropionate (7.1g, 99.1%, 0.02mol) was dissolved in 15mL under nitrogen protection Add 2-cyanophenol (2.6g, 0.022mol) and anhydrous potassium carbonate (4.2g, 0.03mol) to N,N-dimethylformamide, heat up to 80°C and react for 3h, HPLC shows that the reaction of raw materials is complete .
[0059] The reaction mixture was cooled and concentrated under reduced pressure (using a water bath temperature of 80° C.) to obtain a light yellow solid, which was dissolved in toluene (40 mL) and washed with 20 mL of water to obtain 54.2 g of toluene solution, which contained 2-{ 7.12g of methyl 2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3,3-dimethoxypropionate, (E)-2-{2- Methyl [6-(2-cyanophenoxy)pyrimidin-4-yloxy]ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com