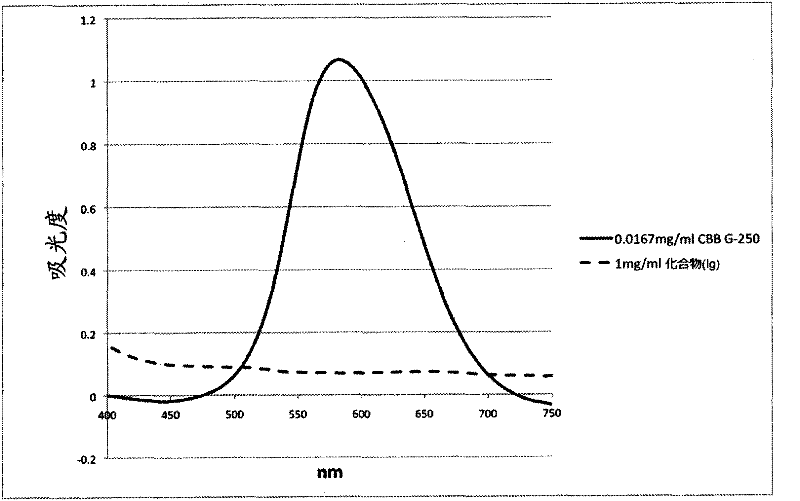
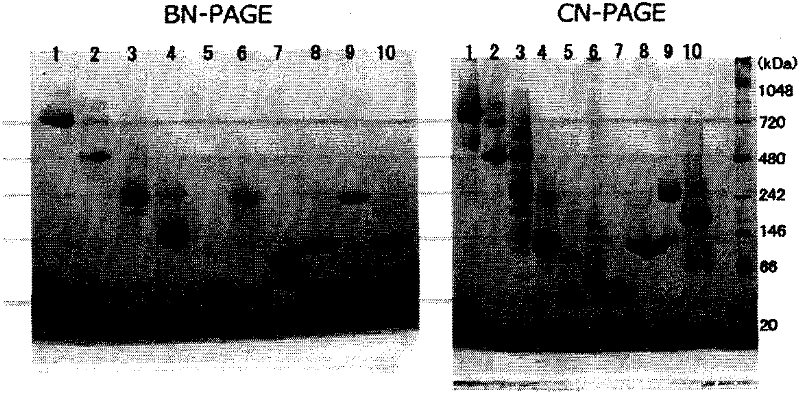
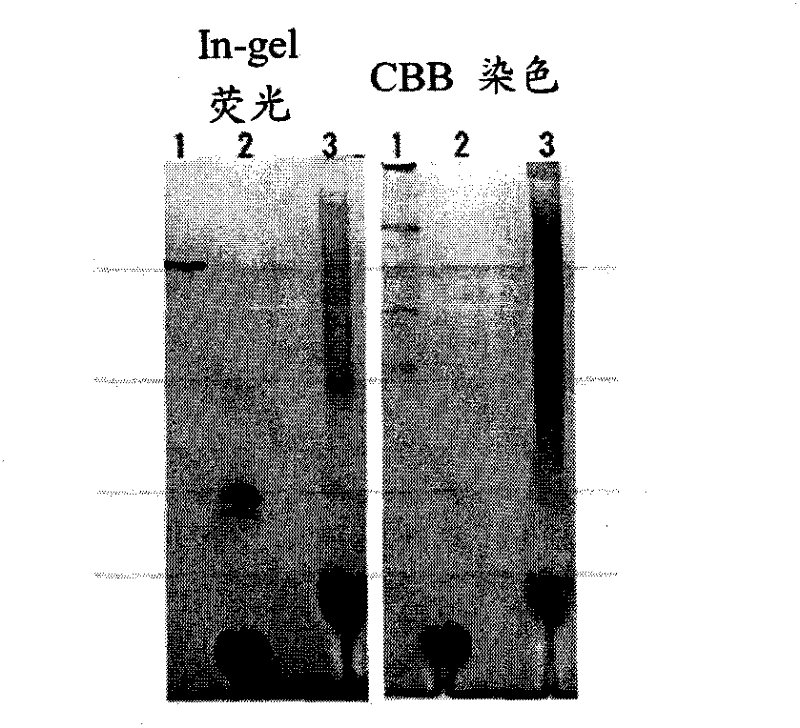
Novel Clear Native electrophoresis method utilizing aromatic sulfonic acid compound
A compound and electrophoresis technology, applied in the fields of peptide preparation, chemical instruments and methods, organic chemistry, etc., can solve the problem of the absence of multimeric proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0182] Samples can be prepared by known methods. For example, a sample can be prepared by mixing the above protein sample in a buffer composition containing compound (I) and a sample buffer. In addition, a sample can be prepared by adding a reagent for electrophoresis containing compound (I) to a buffer obtained by solubilizing a protein sample, and then adding other components such as glycerin as necessary.
[0183] The protein concentration in the sample varies according to the staining method or detection method after electrophoresis, and cannot be set to a fixed value. When detecting by CBB staining, it is usually about 0.5 μg / well to about 50 μg / well, preferably about 50 μg / well. 1 μg / well to about 10 μg / well. In addition, when preparing a fluorescent protein fusion product and detecting the fluorescence of the protein, it is generally 0.05 μg / well to 1 μg / well, preferably about 0.1 μg / well to about 0.5 μg / well. In addition, when performing silver staining and detection...
Embodiment 1
[0220] 1. Production of compounds represented by general formula (Ig)
[0221] At 0°C, in 12.0 g (14.1 mmol) of (Benzenemethanaminium), N-[4-[[4-[(4-ethoxyphenyl)amino]phenyl][4-[ Ethyl[(3-sulfophenyl)methyl]amino]phenyl]methylene]-2,5-cyclohexadiene-1-ylidene]-N-ethyl-3-sulfo-hydrogen Oxide, inner salt, monosodium salt) (CBB G250) were added methanol (60 ml) and 2.65 g of sodium cyanoborohydride (sodium cyanoborohydride) (42.3 mmol), and stirred at room temperature for 1.5 hours.
[0222] After the reaction solution was concentrated under reduced pressure, it was recrystallized by mixing and dissolving diethyl ether and methanol to obtain 8.95 g of 3,3'-(4,4'-((4-(4-ethoxybenzene Amino)phenyl)methylene)bis(3-methyl-4,1-phenylene))bis(ethylazanediyl)bis(methylene)dibenzenesulfonate ( Hereinafter, it is referred to as compound Ig) (yield 73%).
[0223] The NMR analysis results of the obtained compound (Ig) are shown below. 1 H NMR (270MHz, CD 3 OD): δ7.65-7.85(m, 4H), 7.37...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com