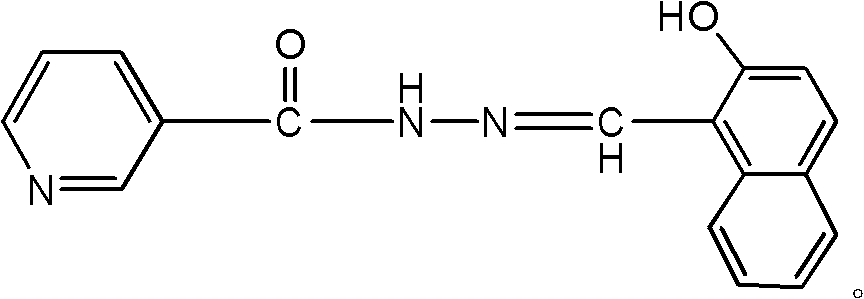
2-hydroxyl-1-naphthalene-3-pyridinecarbonylhydrazone and preparation method and applications thereof
A technology of pyridinecarboylhydrazone and pyridinecarboylhydrazide, which is applied in the field of 2-hydroxy-1-naphthalene-3-pyridinecarboylhydrazone and its preparation, can solve the problems of unscreened herbicidal activity and low cost, and achieve the goal of preparing The method is simple, the cost is low, and the effect of high water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0013] Example: Add 10mmol of 3-pyridinecarbohydrazide and 20-30ml of methanol to the flask, stir under reflux to dissolve the hydrazide completely, then add 10mmol of 2-hydroxyl-1-naphthaldehyde, and immediately a yellow precipitate is formed. React for 2-3 hours, cool to room temperature, and filter with suction to obtain the crude product of acylhydrazone. Recrystallize with a mixed solvent of absolute ethanol and DMF at a volume ratio of 1:1 to obtain yellow crystals. Then wash it with ethanol, dry it, put it in a vacuum desiccator and weigh it to a constant weight to get the pure product. Yield: 80%.
[0014] Through infrared spectrum analysis and NMR analysis, the results are as follows:
[0015] Infrared spectrum (KBr, cm -1 ): υ(OH), 3380, υ(C=O), 1701, υ(C=N), 1655.
[0016] 1 H NMR (d1-DMSO, ppm): δ7.63-9.17 (m, 4H, C 5 h 4 N-), 7.21-7.86 (m, 6H, C 10 h 6 -), 5.00 (s, 1H, -OH), 8.00 (s, 1H, -NH), 8.10 (s, 1H, CH=N). 13 C NMR (d1-DMSO, ppm): δ163.0 (C=O), 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com