Substituted quinazolines as blood platelet lowering agents
A technology of solvates, compounds, applied in the field of substituted quinazolines as platelet reducing agents, able to solve problems such as unrecognized anti-megakaryocyte potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
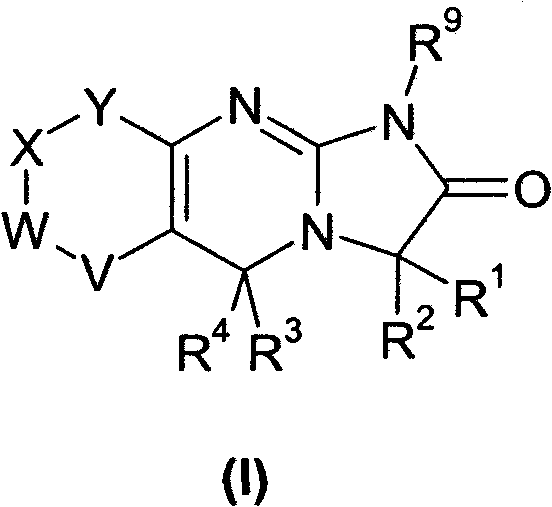
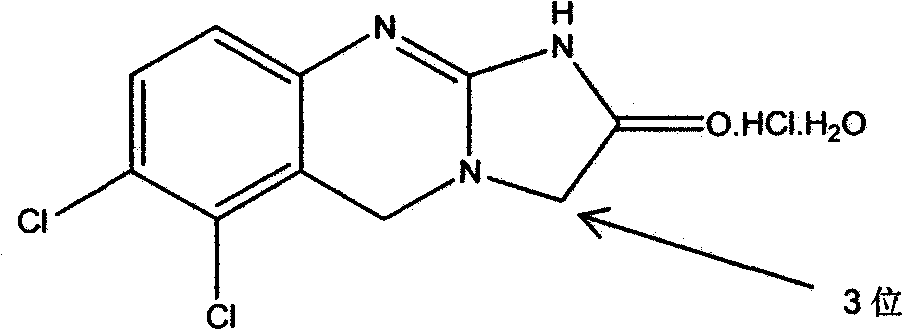
[0121] The present invention relates to novel 3- or 5-substituted analogs of the established platelet reducing agent anagrelide. Substitutions at the 3- or adjacent 5-position of the anagrelide molecule were expected to block or impede major sites of metabolism and potentially prevent the formation of the highly potent PDE III inhibitor 3-OH anagrelide, while unexpectedly Surprisingly, it was found that substitution at the 1-position abolishes PDE III inhibition. The compounds of the present invention retain the antimegakaryocyte properties (and thus the thrombocytopenic activity) of the parent drug molecule, but have reduced PDE III inhibitory properties and therefore have a lower potential for adverse cardiovascular and antiaggregative side effects. They also have the potential to improve the pharmacokinetic profile as a result of inhibited metabolism.
[0122] Pharmaceutically acceptable acid addition salts of certain compounds of formula (I) can also be prepared in a conv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com