Method for producing polycrystalline silicon by utilizing sodium fluosilicate byproduct of phosphate fertilizer
A technology of sodium fluorosilicate and by-products, which is applied in the field of polysilicon production, can solve problems such as non-compliance with green development, environmental pollution, and reduced conversion rate of raw materials, and achieve the effects of saving raw materials, low impurity content, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
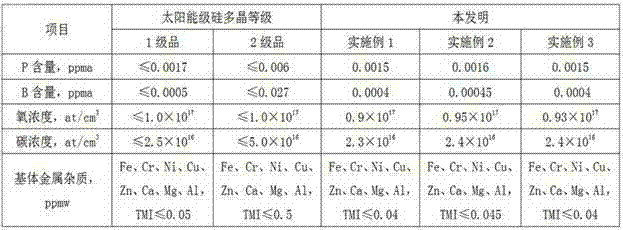
Examples
Embodiment 1
[0026] The production method of this embodiment is as follows:
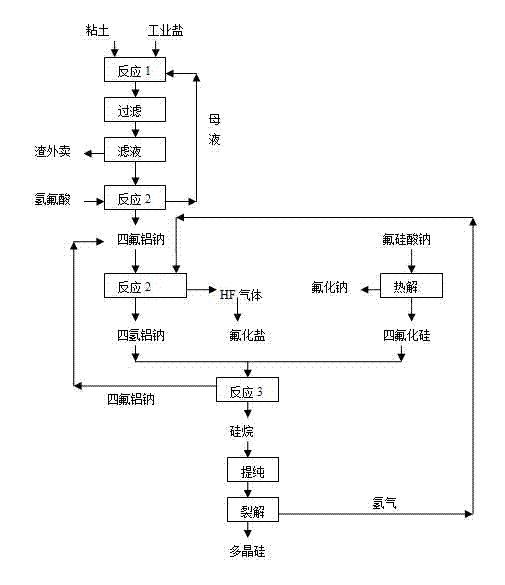
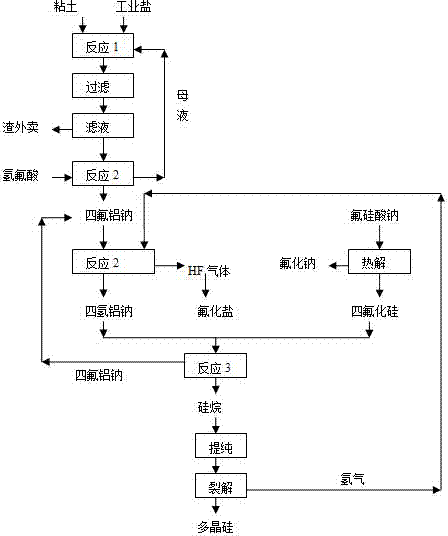
[0027] (1) Using clay, industrial salt, and hydrochloric acid as raw materials, leaching at 70℃ for 0.5h according to the ratio of Na / Al=0.95, and then filtering, the filtrate reacts with hydrofluoric acid to produce sodium tetrafluoroaluminum, and the mother liquor is returned ;
[0028] (2) The above-prepared sodium tetrafluoroaluminum and hydrogen are reacted at 600°C under the action of a palladium catalyst to produce sodium tetrahydro-aluminum according to the theoretical ratio;
[0029] (3) At the same time, pyrolyze sodium fluorosilicate, a byproduct of phosphate fertilizer required by the theoretical ratio, at 700°C to generate sodium fluoride and silicon tetrafluoride gas;
[0030] (4) The mass ratio of 2:1 sodium tetrahydroaluminum and silicon tetrafluoride are reacted in a mixed solvent of 60°C, glycol dimethyl ether and toluene to obtain silane and sodium tetrafluoroaluminum, sodium tetrafluoroaluminum Recyc...
Embodiment 2
[0035] The production method of this embodiment is as follows:
[0036] (1) Using clay, industrial salt, and hydrochloric acid as raw materials, leaching at 80℃ for 1 hour according to the ratio of Na / Al=1.0, then filtering, the filtrate reacts with hydrofluoric acid to produce sodium tetrafluoroaluminum, and the mother liquor is returned ;
[0037] (2) The above-prepared sodium tetrafluoroaluminum and hydrogen are reacted at 400°C under the action of a nickel catalyst according to the theoretical ratio to generate sodium tetrahydroaluminum;
[0038] (3) At the same time, pyrolyze sodium fluorosilicate, a byproduct of phosphate fertilizer required by the theoretical ratio, at 770°C to generate sodium fluoride and silicon tetrafluoride gas;
[0039] (4) The reaction of sodium tetrahydroaluminum sodium and silicon tetrafluoride with a mass ratio of 3:1 in a mixed solvent of 80°C, glycol dimethyl ether and toluene to obtain silane, sodium tetrafluoroaluminum, and tetrafluoroaluminum Sod...
Embodiment 3
[0044] The production method of this embodiment is as follows:
[0045] (1) Using clay, industrial salt, and hydrochloric acid as raw materials, leaching at 90°C for 1 hour according to the ratio of Na / Al=1.1, then filtering, the filtrate reacts with hydrofluoric acid to produce sodium tetrafluoroaluminum, and the mother liquor is returned ;
[0046] (2) The above-prepared sodium tetrafluoroaluminum and hydrogen are reacted to form sodium tetrahydro-aluminum at 200°C under the action of a nickel catalyst according to the theoretical ratio;
[0047] (3) At the same time, pyrolyze sodium fluorosilicate, a byproduct of phosphate fertilizer required by the theoretical ratio, at 900°C to generate sodium fluoride and silicon tetrafluoride gas;
[0048] (4) The reaction of sodium tetrahydroaluminum sodium and silicon tetrafluoride with a mass ratio of 4:1 in a mixed solvent of 100°C, glycol dimethyl ether and toluene to obtain silane, sodium tetrafluoroaluminum, and aluminum tetrafluoride S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com