Methods, dosage forms, and kits for administering ziprasidone without food
A technology of ziprasidone and dosage forms, which is applied in the direction of pharmaceutical formulas, drug combinations, medical preparations containing active ingredients, etc., and can solve problems such as poor compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] Ziprasidone dissolved in SBECD
[0173] Preparation of Ziprasidone Test Formulation: The ziprasidone test formulation comprises ziprasidone mesylate-SBECD lyophilized powder combined with HPMCAS in a mass ratio of about 1:6:2. The formulation was prepared according to the procedure given below: SBECD was charged into a flask containing water for injection (WFI) and heated until a solution was obtained. Subsequently, ziprasidone mesylate was added to the flask and heated until a solution was obtained. The solution was cooled and kept between 35 and 40C, then filtered through a 0.45 micron Kleenpak Ultipro N66 filter into a holding vessel. Subsequently, the solution was transferred to a dish. The solution was frozen at at least -40°C before the lyophilization cycle was initiated. Take a few weeks to warm up. The final drying cycle reduces the moisture content to less than 2%. The lyophilized powder is subsequently milled. 176.2 mg of milled lyophilized powder (equiv...
Embodiment 2
[0186] Ziprasidone nanoparticles
[0187] Preparation of Candidate Formulations: The test formulation, referred to herein as Formulation B, contained ziprasidone free base in nanoparticulate form. The formulation was prepared according to the procedure given below: A coarse suspension was prepared by placing 8.85 gm of ziprasidone free base into a 100 ml milling chamber containing 48.89 gm of milling media (500 micron polystyrene beads) liquid. To this was added 4.2ml of each of the following solutions 10% of F108 solution, 80 and 5% lecithin solutions. Additionally, 23.8 ml of water for injection was added to the milling chamber. The above mixture was stirred until a homogeneous suspension was obtained. Subsequently, the suspension was milled in a Nanomill-1 (Manufacturer ElanDrug Delivery, Inc.) at 2100 RPM for 30 minutes, with the temperature maintained at 4°C during milling. The resulting suspension was vacuum filtered to remove the milling media. An appropriate v...
Embodiment 3
[0198] Ziprasidone HCl
[0199] Ziprasidone coated crystals containing 35% active ziprasidone hydrochloride monohydrate coated with the precipitation inhibiting polymer HPMCAS were prepared as described in the aforementioned US Patent Application Publication 2007 / 0190129.
[0200] The test ziprasidone formulation of Example 3 (referred to as "Formulation C") was administered in the form of a powder-in-capsule (1 x 40 mg). The test drug information is shown in Table 5.
[0201] Table 5 Information on Study Drugs
[0202]
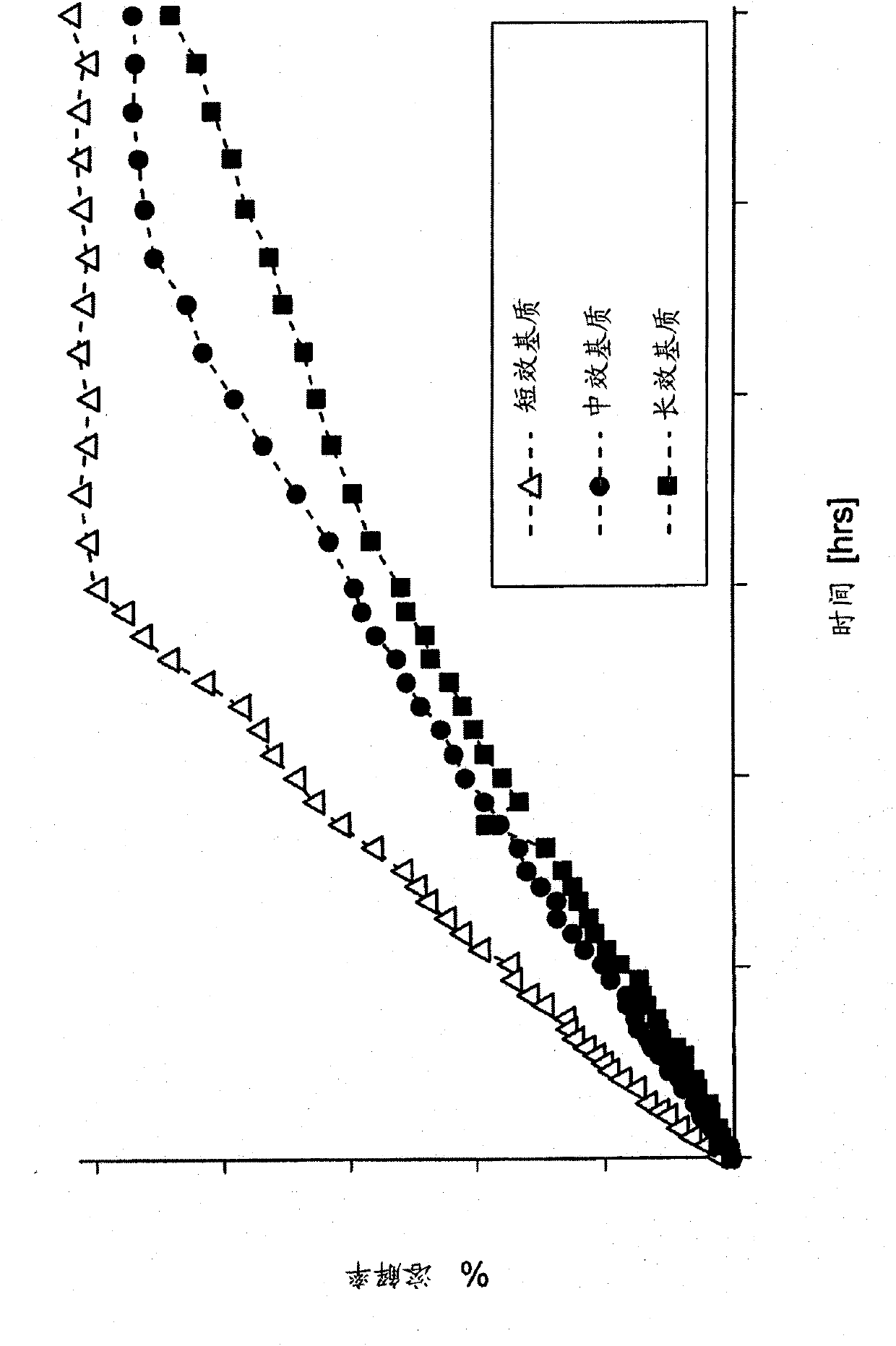
[0203] Results: The PK parameters after a single dose of the test formulations in the fed and fasted state, as well as after a single dose of the commercially available capsules in the fed state are summarized in Table 6. These data show the fasted AUC of the test formulation inf is the fed AUC of the test formulation inf 57% of and the fasting AUC of the test formulation inf It is 62% of the commercially available capsules fed state.
[0204] Table ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com