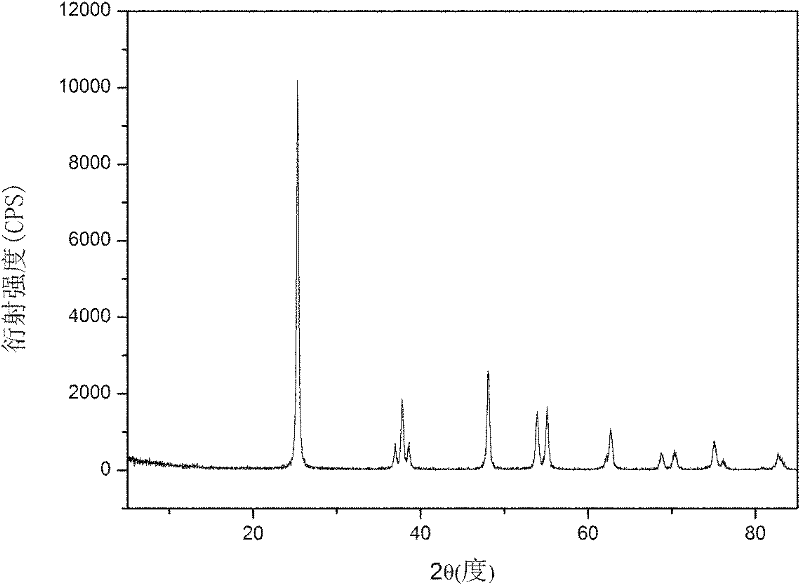
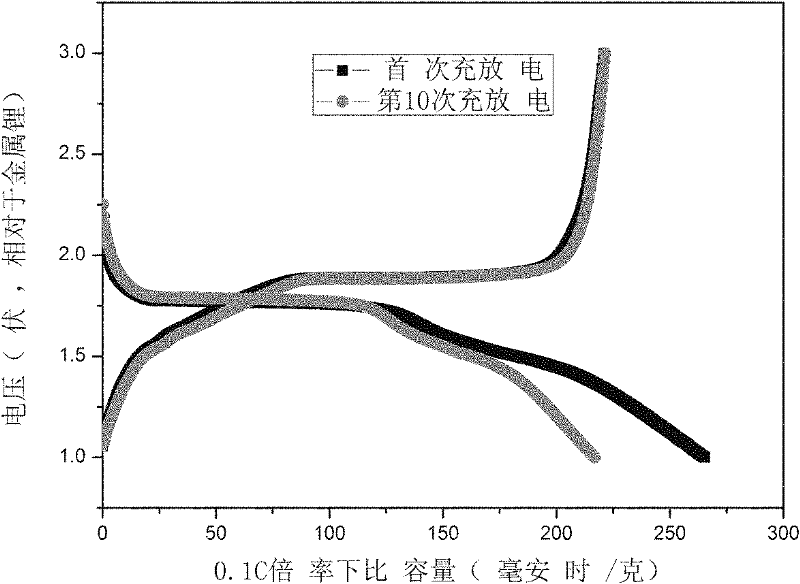
Method for preparing nano-TiO2 serving as cathode material of lithium ion battery
A technology for lithium-ion batteries and negative electrode materials, applied to battery electrodes, circuits, electrical components, etc., can solve the problems of unsuitability for large-scale production, long reaction time, and high raw material costs, and achieve industrial production convenience, short reaction cycle, and equipment simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The titanium source is 15 grams of chemically pure titanium sulfate, which is dissolved in water. In the experiment, the pH is adjusted to 3 with 10wt.% ammonia water, and the precipitate of titanium is obtained by filtration; the precipitate is beaten with distilled water, and hydrogen peroxide solution is added. The molar ratio is 1. During the reaction, 0.5mol / L sodium hydroxide is used to adjust the pH of the solution to 7, and then react in a stirred reactor at 20°C. After 10 minutes, filter to obtain a titanium solution, and then pour it into a 1000ml beaker. ; Add 10wt.% sodium hydroxide solution to the titanium solution, the molar ratio of sodium hydroxide to titanium is 1; place the beaker on an electric furnace and heat it to 80°C, stir the stirring paddle continuously, and filter to obtain a precipitate after 10 minutes thing. The precipitate was treated with 1wt.% HNO 3 Washing 2 times, and then washing 2 times with distilled water to obtain nanostructured ...
Embodiment 2
[0039] The titanium source is ilmenite at 120°C, the ratio of acid to mineral is 1.2:1, 5 grams of hydrolyzed high-titanium slag after hydrochloric acid leaching for 2 hours, beat the titanium slag with distilled water, add hydrogen peroxide solution, the molar ratio of hydrogen peroxide and titanium The ratio is 6: 1, and the pH of the solution adjusted with 30wt.% ammonia water is 10.5 and reacted in a stirred reactor at 35° C. for 50 minutes, filtered to obtain the titanium solution, and poured into a 1000ml beaker; Lithium hydroxide solution was added into the solution, and the molar ratio of lithium hydroxide to titanium was 10:1; the beaker was placed on an electric furnace and heated to 140° C., the stirring paddle was constantly stirred, and the precipitate was filtered after 1 hour. The precipitate was treated with 2wt.% HNO 3 Washing 2 times, and then washing 2 times with distilled water to obtain nanostructured TiO 2 The precursor; the precursor was calcined at 600...
Embodiment 3
[0041] The titanium source is 10 grams of chemically pure tetrabutyl titanate, which is dissolved in water to obtain a white flocculent precipitate. Add sodium peroxide solution, the molar ratio of sodium peroxide and titanium is 5: 1, adjust the pH of the solution to 10 with 10wt.% ammonia water in the experiment, react in a stirred reactor at 40°C for 30 minutes, and filter to obtain titanium The solution is poured into a three-necked flask with a volume of 1000ml; lithium hydroxide solution is added to the titanium solution, and the molar ratio of lithium hydroxide to titanium is 10:1; the three-necked flask is placed in an oil bath and heated to 120°C. Stirring was continued with a stirring bar, and a precipitate was obtained by filtration after 1 hour. The precipitate was washed twice with 1wt.% HCl, and then washed twice with distilled water to obtain nanostructured TiO 2 The precursor; the precursor was calcined at 120°C for 10 hours to obtain nano-TiO 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com