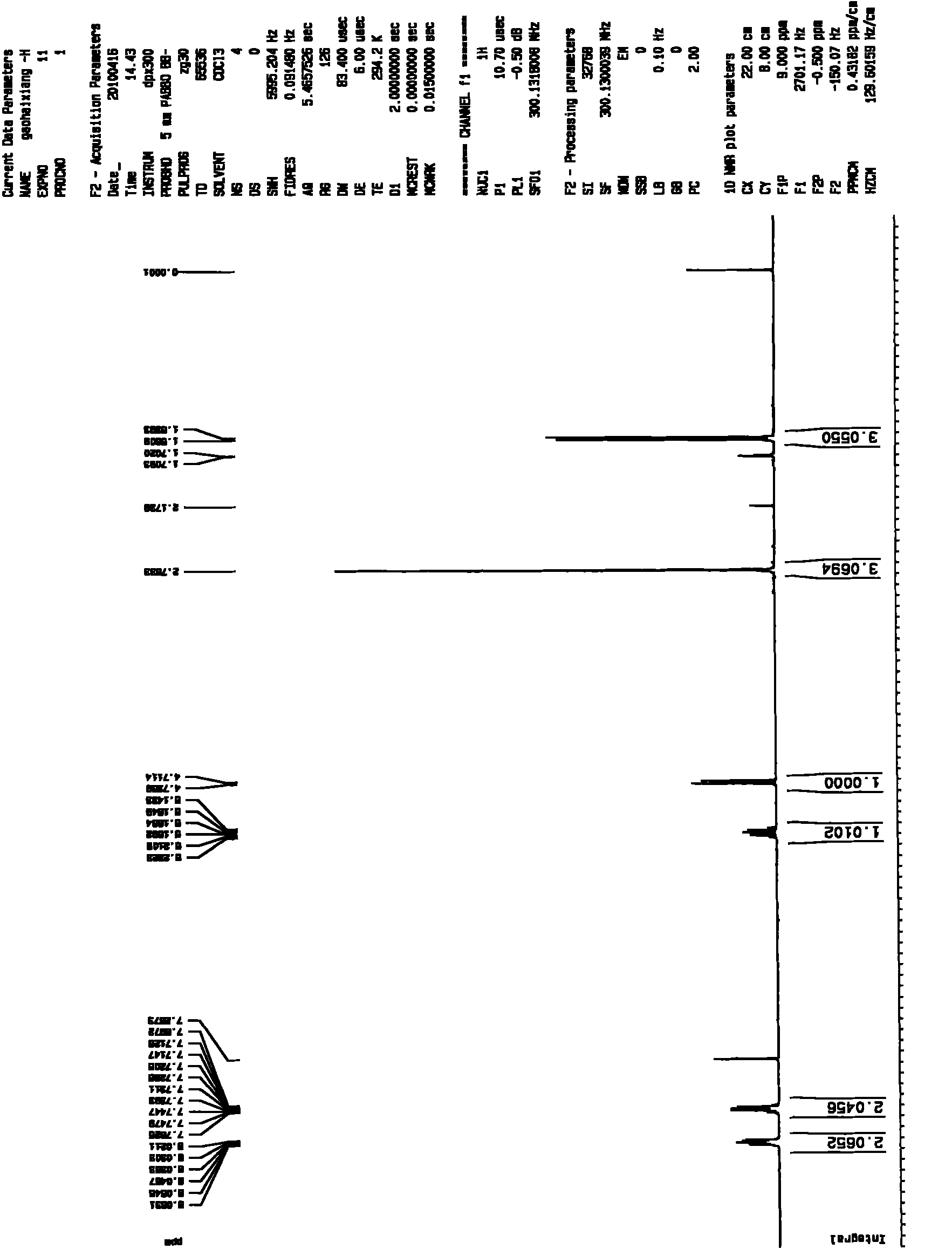Preparation method of 3-methyl-2-ethanol based quinoxaline
A technology for quinoxaline and acetylquinoxaline is applied in the field of preparation of 3-methyl-2-ethanol quinoxaline, can solve problems such as human health hazards and industrial development obstacles, and achieves concise synthesis steps and convenient post-processing. , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
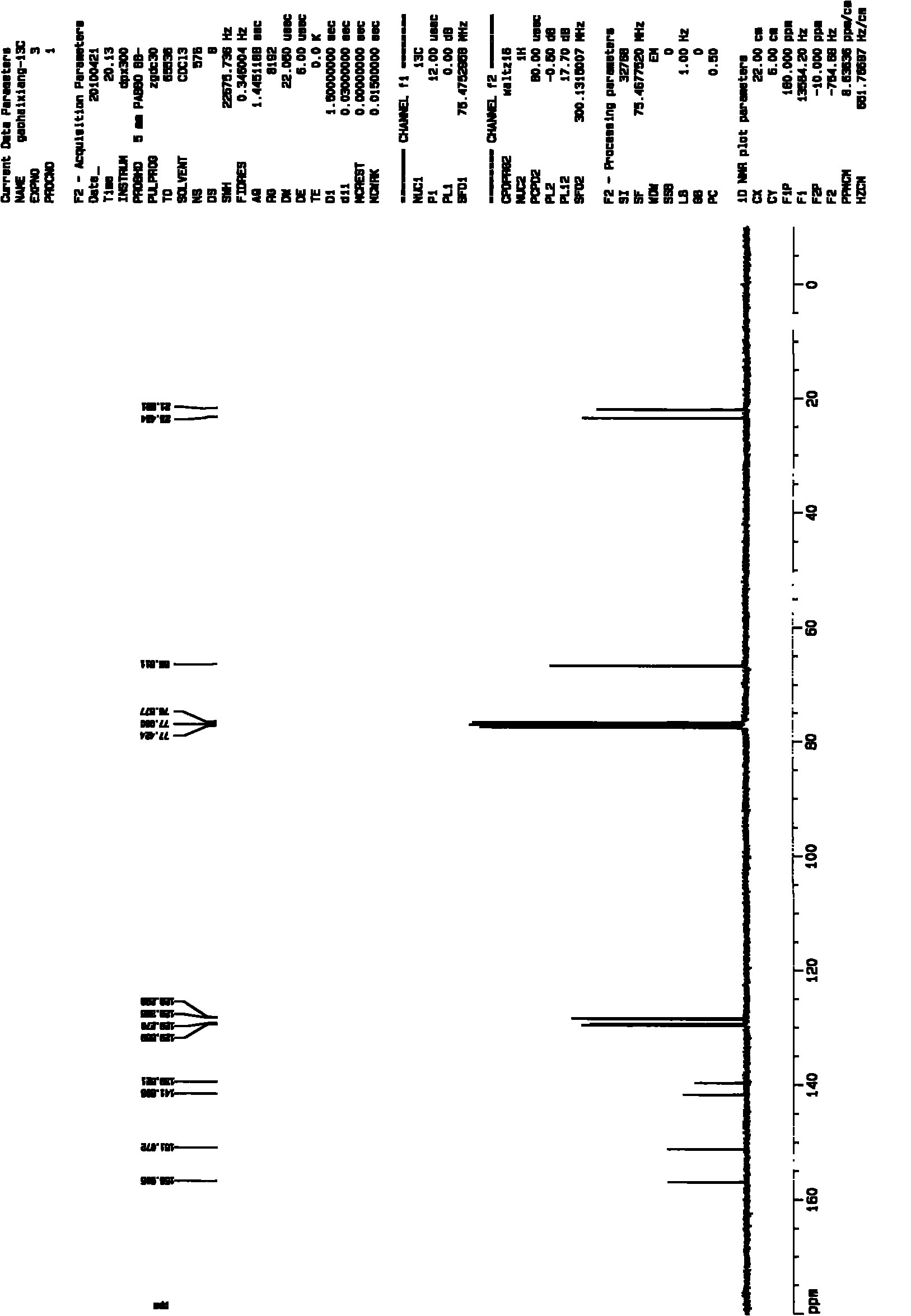
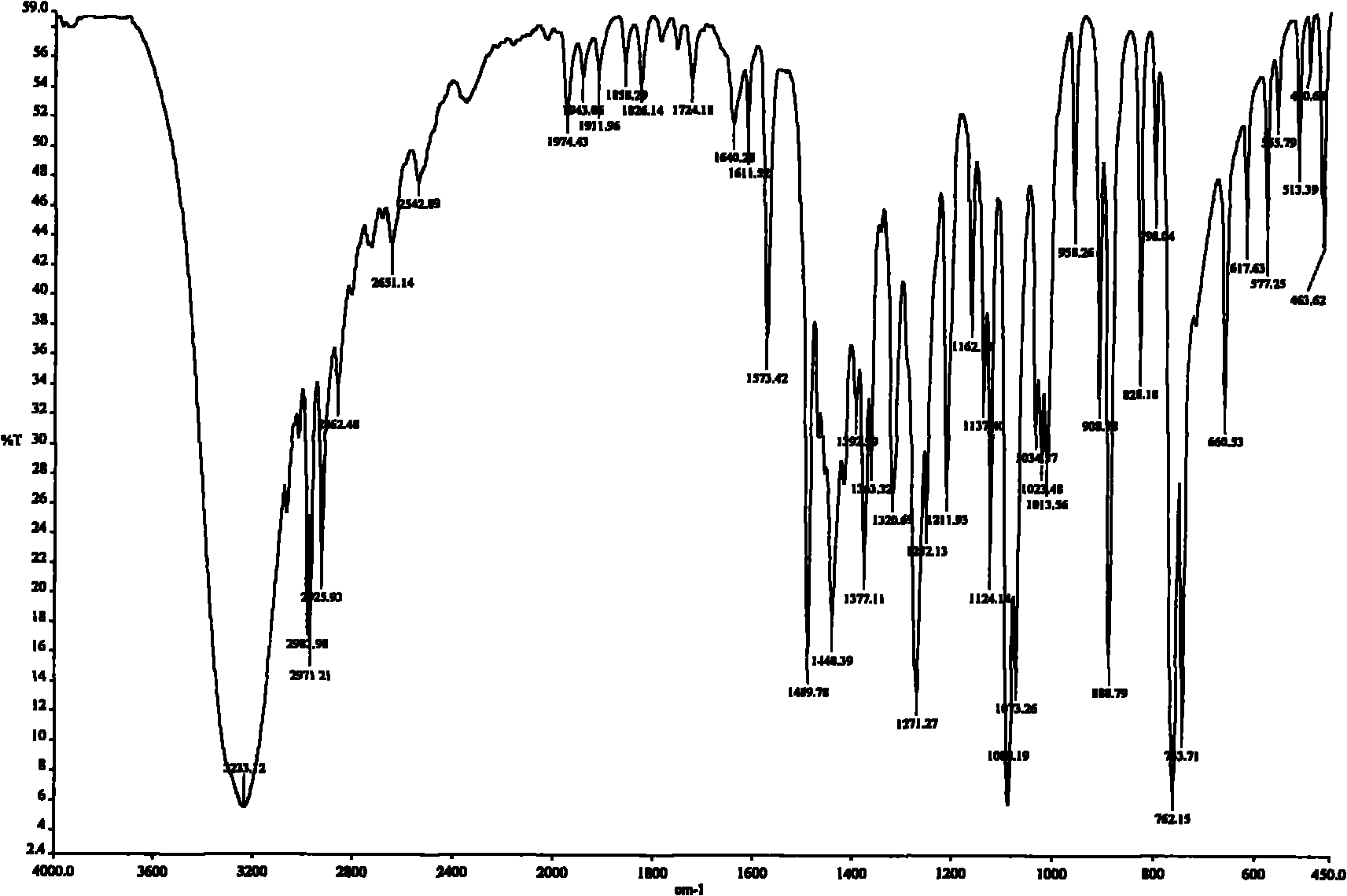
[0029] The preparation of embodiment 1,3-methyl-2-ethanolylquinoxaline
[0030] (1) Preparation of 3-methyl-2-acetylquinoxaline
[0031] Weigh 3g of acetylmequine, add 40mL of tetrahydrofuran, 20mL of 100% ethanol, 20mL of distilled water, stir and heat to dissolve at 30°C, then add 9g of sodium hyposulfite, the molar ratio of acetylmethoquine to sodium hyposulfite is 1:3.75, keep the solution temperature at 50 °C, reacted for 2 hours and then cooled. Most of the solvent was evaporated by rotary evaporation, 20 mL of distilled water was added, extracted three times with 20 mL of chloroform, and the organic phases were combined. The organic phase was dried with anhydrous sodium sulfate, and the filtrate was obtained by suction filtration, and 2.2 g of yellowish-brown 3-methyl-2-acetylquinoxaline crude product was obtained by suction filtration, which was set aside.
[0032] (2) Preparation of 3-methyl-2-ethanolylquinoxaline
[0033] The crude 3-methyl-2-acetylquinoxaline obt...
Embodiment 2
[0034] The preparation of embodiment 2,3-methyl-2-ethanolylquinoxaline
[0035] (1) Preparation of 3-methyl-2-acetylquinoxaline
[0036] Weigh 4g of methquine, add 45mL of tetrahydrofuran, 25mL of 100% ethanol, 25mL of distilled water, stir and heat to dissolve at 30°C, then add 10g of sodium hyposulfite, the molar ratio of acetylmethoquine to sodium hyposulfite is 1:3.15, and keep the solution temperature at 55 °C, reacted for 2 hours and then cooled. Most of the solvent was evaporated by rotary evaporation, 25 mL of distilled water was added, extracted three times with 25 mL of chloroform, and the organic phases were combined. After drying the organic phase with anhydrous sodium sulfate, the filtrate was obtained by suction filtration, and 2.7 g of yellowish brown 3-methyl-2-acetylquinoxaline crude product was obtained by suction filtration, which was set aside.
[0037] (2) Preparation of 3-methyl-2-ethanolylquinoxaline
[0038] The crude 3-methyl-2-acetylquinoxaline obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com