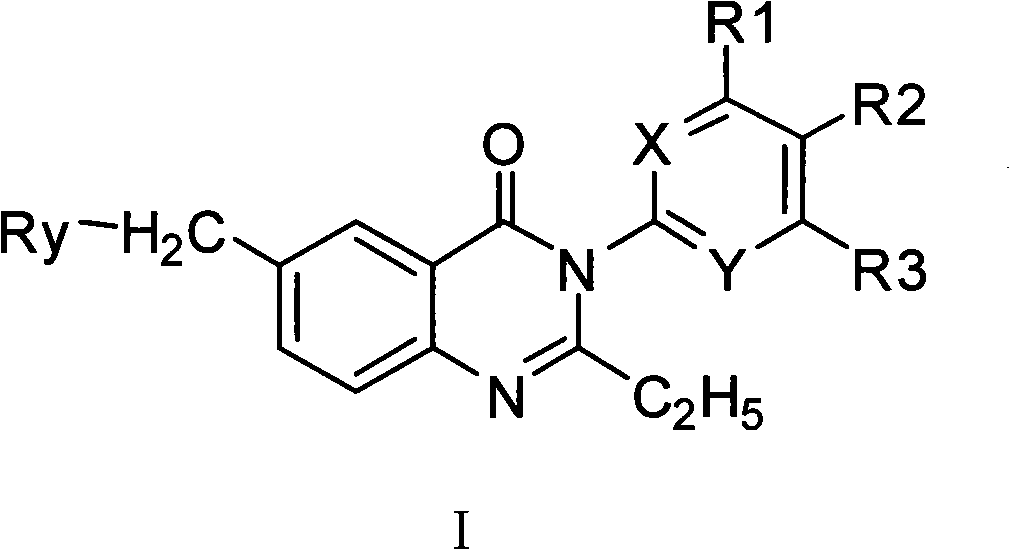
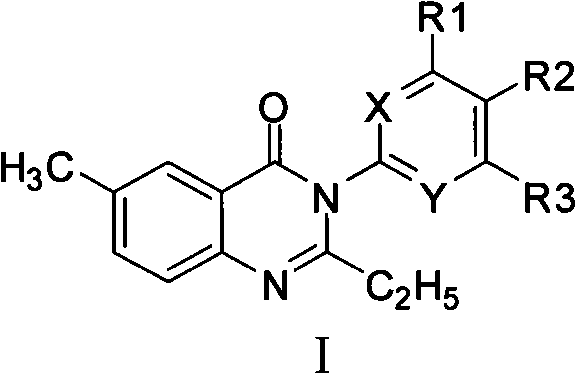
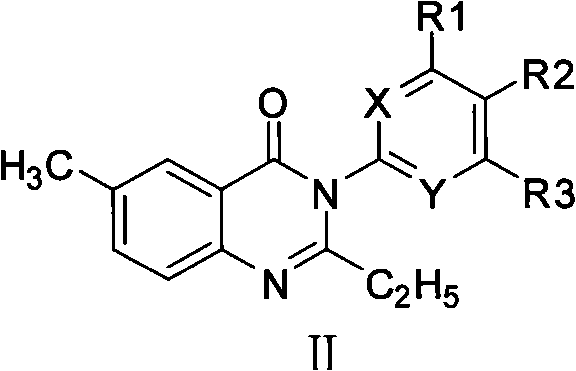
Quinazol derivative, preparation method and application thereof
A kind of technology of quinazolinone and derivatives, applied in the field of pharmaceutical compounds, can solve problems such as large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The synthetic method of 3-substituted-4 (3H)-quinazolone compound (compound 1), comprises the following steps:
[0104]
[0105] Steps:
[0106] (1) Preparation of 2-ethyl 6-methylbenzoxazin-4-one (intermediate 2)
[0107] Take 0.04mol of 2-amino-6-methylbenzoic acid and 50ml of propionic anhydride, heat, stir and reflux for 3h-5h, stop the reaction, let cool to room temperature, place in the refrigerator overnight, filter and precipitate white flaky crystals to obtain the crude product. Recrystallized from absolute ethanol to obtain light yellow intermediate 2 (5.24g).
[0108] (2) Preparation of Target 3 (Compound 1)
[0109] Take intermediate 2 (1.5g, 0.005mol), equimolar 3-substituted 4-chloroaniline 0.635g, dissolve in 10mlN, a mixed solution of N dimethylformamide (DMF) and 50ml of tetrahydrofuran, add 0.5g of Catalyst dicyclohexylcarbodiimide (DCC), stirred and refluxed for 10h. After filtration, the filtrate was rotary evaporated to remove the mixed solve...
Embodiment 2-7
[0110] Embodiment 2-7 (preparation of compound 2-7)
[0111] The method is the same as in Example 1, but the 3-substitute of compound 2-8 of step (2) is different from the target object, see the following table:,
[0112]
[0113]
Embodiment 8
[0115] The synthetic method of 6-substituted-4 (3H)-quinazolone compound (compound 8), comprises the following steps:
[0116]
[0117] Steps:
[0118] (1) Preparation of 2-ethyl 6-methylbenzoxazin-4-one (intermediate 2)
[0119] Take 6.0g (0.04mol) of 2-amino-6methylbenzoic acid and 50ml of propionic anhydride, heat, stir and reflux for 3h-5h, stop the reaction, let it cool to room temperature 25°C, put it in the refrigerator overnight, filter and precipitate white flaky crystals , 5.82g crude product was obtained. Using 8ml of absolute ethanol, recrystallized to obtain 25.24g of light yellow compound.
[0120] (2) Preparation of 2-ethyl 6-methyl-4-(3H)-quinazolinone (intermediate 3)
[0121] Take compound 2 (0.043mol) and 30ml formamide and stir for 7h. Cool to room temperature 25°C, filter out tan needle crystals, wash with a small amount of water (10ml×3), and dry to obtain compound 36.10g.
[0122] (3) Preparation of 2-ethyl 6-bromomethyl-4-(3H)-quinazolinone (int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com