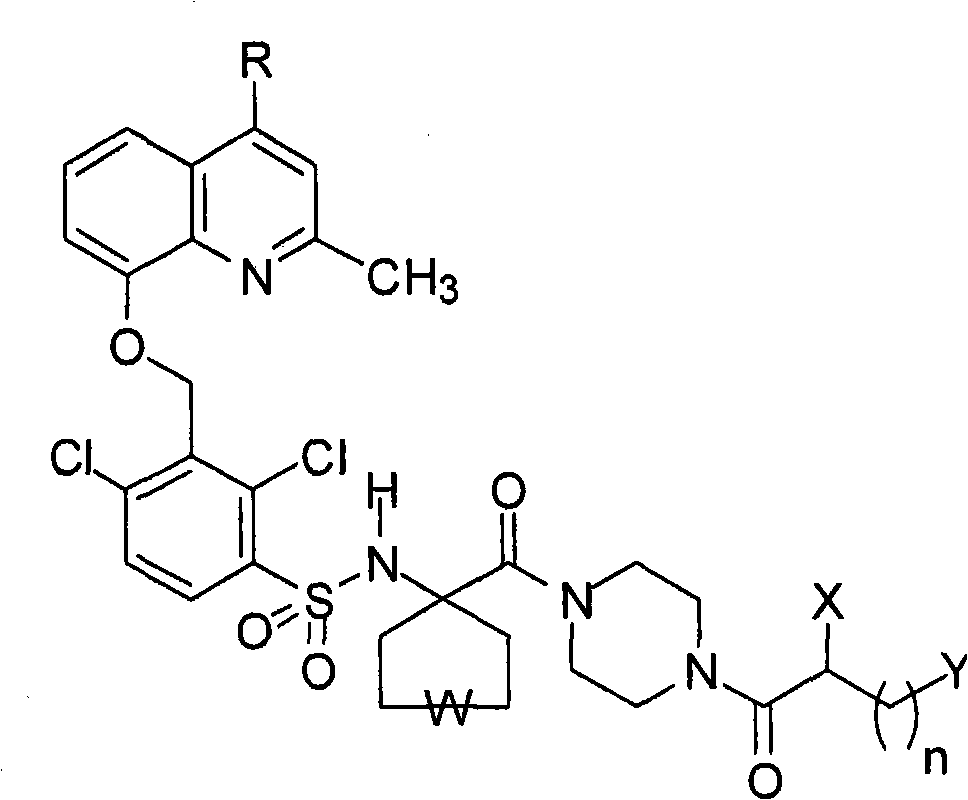
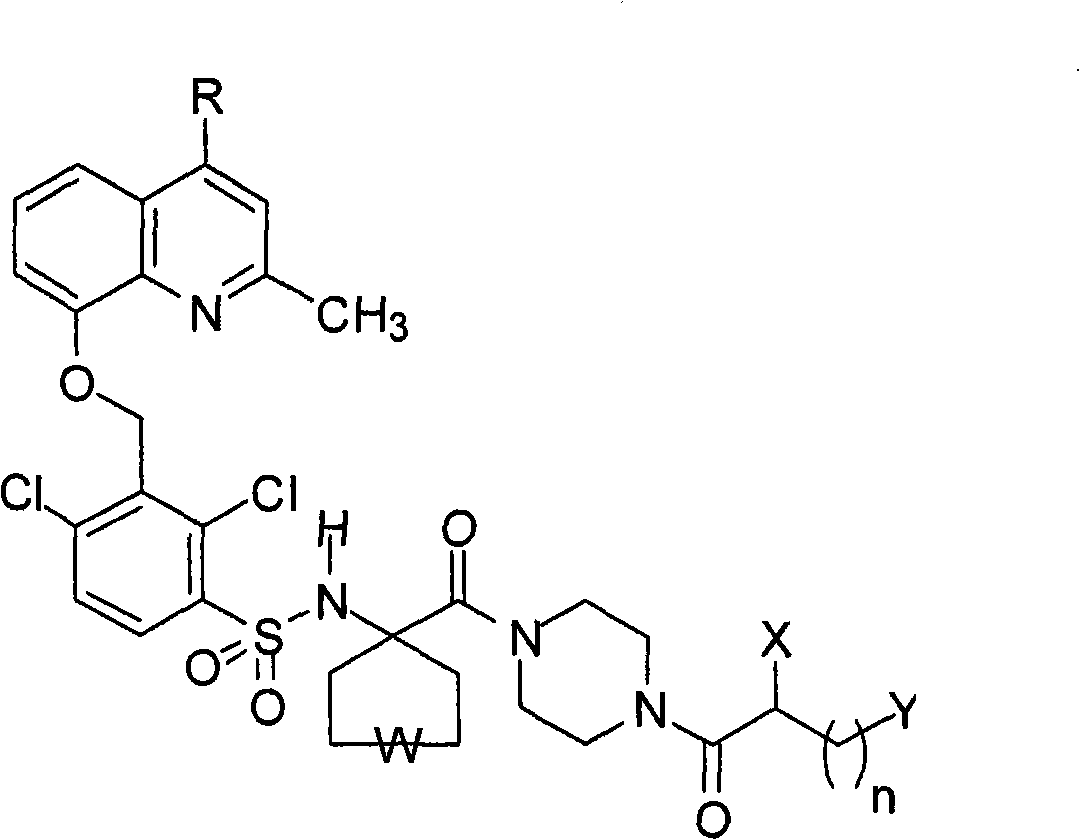
Pharmaceutical compositions containing bradykinin antagonists and hyaluronic acid, and uses thereof
A technology of hyaluronic acid and composition, applied in the field of pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Hyaluronic acid, sodium salt, its average molecular weight is 6 million Daltons, 10 mg, MEN1613 20.5 mg, in saline solution (0.9% NaCl), add appropriate amount of 0.1N HCl to pH 4.5, add appropriate amount of water to 1 ml. This solution was placed in a pre-filled 2.25ml syringe.
Embodiment 2
[0072] Hyaluronic acid, sodium salt, its average molecular weight is 6 million Daltons, 10 mg, MEN1613 20.5 mg, in a saline solution (0.9% NaCl) containing a phosphate buffer (NaCl 2 HPO 4 0.16 mg NaH 2 PO 4 0.04mg), add appropriate amount of water to 1ml. This solution was placed in a pre-filled 2.25ml syringe.
Embodiment 3
[0074] Hyaluronic acid, sodium salt, its average molecular weight is 6 million Daltons, 10mg, MEN161320.2mg, in saline solution (0.9% NaCl), add appropriate amount of 0.1N HCl to pH 4.5, add appropriate amount of water to 1ml. This solution was placed in a pre-filled 2.25ml syringe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com