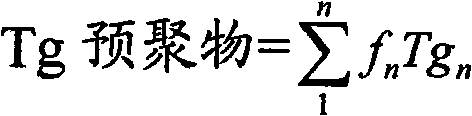Polyacrylic moisture curable copolymer
A polyacrylic and acrylic technology, applied in the field of polymers, can solve problems such as expensive manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 - Synthesis of Functionalized Moisture Curable Prepolymer
[0029] 175g butyl acrylate (Tg-55°C), 110g methyl acrylate (Tg 8°C), 13g lauryl methacrylate (Tg-65°C), 3g methyl methacrylate (Tg 105°C) and 1g hydroxyethyl acrylate The ester (Tg - 15°C) was added to the mixing tank along with 1.5 g dodecanethiol and 0.3 g benzoyl peroxide and mechanically stirred for 1 minute. 100 g of tert-butyl acetate was added to the round bottom flask and refluxed. Over a period of more than 3 hours, the acrylate-based monomer mixture containing peroxide and thiol was added dropwise, and the mixture was heated to above 110° C. with mechanical stirring. Over a period of 15 minutes, an additional 0.3 g of benzoyl peroxide was added to the flask, followed by maintaining the reaction temperature for 1 hour. Then, 0.1 g of benzoyl peroxide was added over a further 5 minutes and the reaction temperature was maintained for an additional 1 hour. After this time, another 30 g of so...
Embodiment 2
[0031] Example 2 - Synthesis of Coating Type Medium Tg Polyacrylic Polymer
[0032] The butyl acrylate and lauryl methacrylate in Example 1 are replaced with corresponding amounts of methyl methacrylate and methyl acrylate respectively, and the method of Example 1 is repeated to obtain 13 mole fractions of acrylic acid monomers. % methyl acrylate and 33% methyl methacrylate, the monomer percentage weighted prepolymer glass transition temperature is 25°C. The obtained prepolymer had an average molecular weight of 36,000 and an average functionality of 3. When moisture cured, polyacrylic acid has a tensile strength of 147 lbs / linear inch and a linear percent elongation of 565%. The obtained polyacrylic resin was harder than the product of Example 1, but still flowable. The high tensile strength and high cohesive strength of the polyacrylic polymer formed from this prepolymer comes at the expense of the loss of adhesion to glass, aluminum substrates relative to the polyacryl...
Embodiment 3
[0033] Example 3 - Inventive polyacrylate system with high glass transition temperature
[0034] Adopt the methacrylate (methyl methacrylate) that accounts for 11 mole percent of existing acrylate monomer and 73 mole percent of acrylic acid monomer, reduce remaining acrylate polymer appropriately simultaneously, repeat embodiment 1 Methods. The resulting prepolymer had a weighted monomer percent glass transition temperature of 50°C. The isocyanate groups form crosslinked polymers upon moisture curing, producing polymers which are less tacky than the polymer prepared according to Example 2, but which are significantly tougher and harder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com