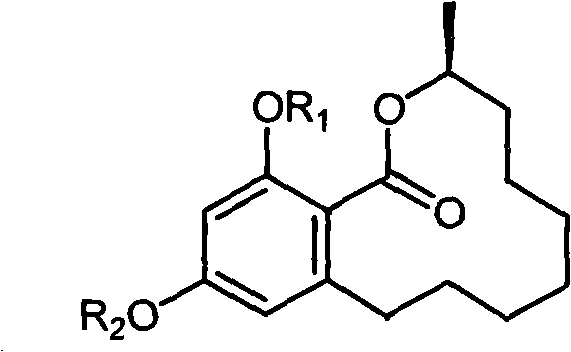
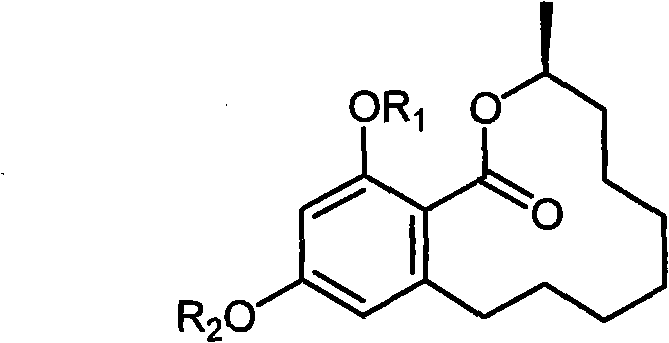
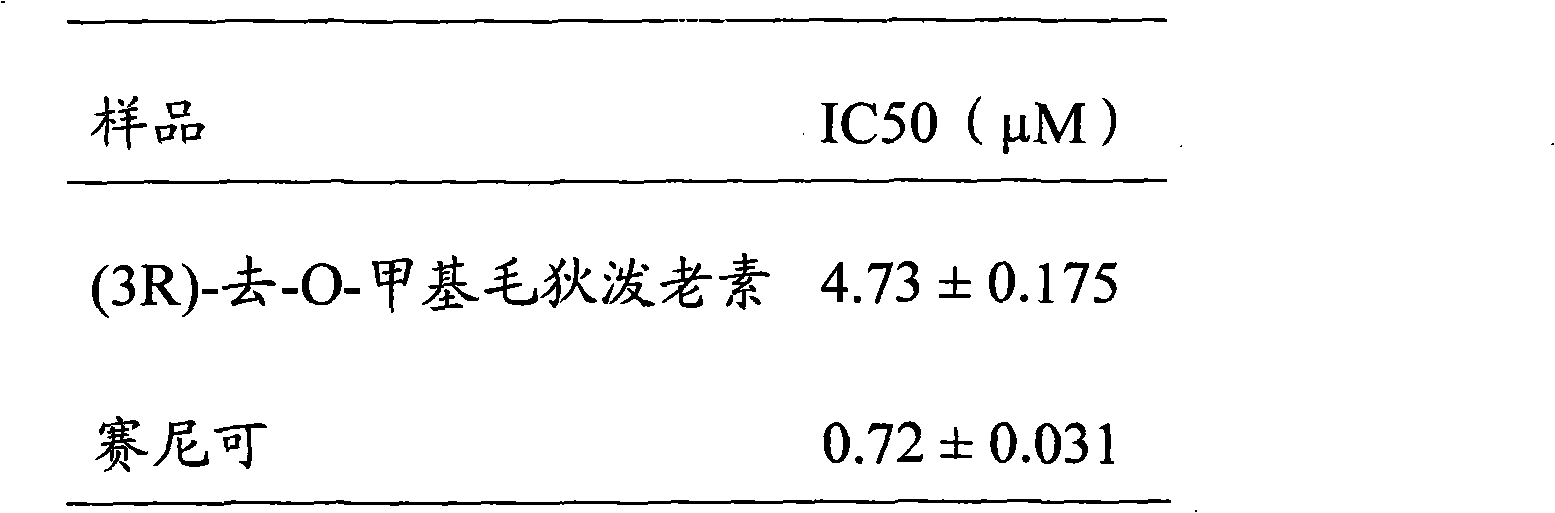
Benzo macrolide compound (3R)- des-O-methyllasiodiplodin, its derivatives and preparation method and use
A technology of methylmadiprene and macrolides, which is applied in the field of benzomacrolides and can solve problems such as no biological activity research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Benzomacrolide Compounds (3R)-Des-O-Methylmadipredine
[0025] (1) Extraction: The dry weight of mangrove mangosteen in China is 3.1kg, and it is extracted 4 times by percolation with 10L of methanol. The extracts are combined and then concentrated under reduced pressure. Suspension 3 times, the resulting extracts were combined and concentrated under reduced pressure to obtain 11 g of petroleum ether extract.
[0026] (2) Separation: 11g of petroleum ether extract was subjected to 100-200 mesh silica gel column chromatography, and petroleum ether / ethyl acetate 100:0→95:5→90:10→70:30→50:50→30:70 → 0:100 gradient elution, the amount of each gradient is 1000ml; among them, 1.2g of the eluted part with a volume ratio of petroleum ether / ethyl acetate of 60:40 is washed with a gradient of petroleum ether / acetone 90:10→80:20→70:30 The amount of each gradient is 200ml; wherein, petroleum ether / acetone volume ratio 80:20 eluted part is subjected to Sephadex LH-...
Embodiment 2
[0029] (3R)-Methylate (R 1 and R 2 are all methyl) prepare
[0030] Weigh 5.0 mg of the (3R)-de-O-methylmaudeprene sample prepared in Example 1 in a 25 mL round bottom flask, add 2 mL of NaOH solution dissolved with 2.5 times the equivalent of methyl iodide, and add an appropriate amount of phase Transfer catalyst tetrabutylammonium bromide, heat reflux in 10mL water / dichloromethane two-phase system for 4 hours, obtain the methylate (R 1 = R 2 =CH 3 ).
[0031] (3R)-De-O-methylmadipredine methylated spectral data are as follows: C 18 h 28 o 4 ; 1 HNMR (300MHz, CDCl 3) 6.24(d, J=2.6Hz, H-5′), 6.21(d, J=2.6Hz, H-3′), 5.18(m, H-3), 3.65, 3.61 (both s, 2×OCH 3 ), 3.26, 2.51 (m, H-10), 1.40~2.49 (H-4~9), 1.37 (d, J=7.0Hz, 3-Me).
Embodiment 3
[0033] (3R)-Acetylate (R 1 and R 2 are all acetyl) preparation
[0034] Weigh 5.0 mg of the (3R)-des-O-methylmaudeprene sample prepared in Example 1 and dissolve it in 4 mL of pyridine solution, add 2 mL of acetic anhydride solution, and react overnight at room temperature to obtain (3R)-des- The acetylated compound of O-methylmadipredrine (R 1 =R 2 =COCH 3 ).
[0035] (3R)-De-O-methylmadipredine acetylate spectral data are as follows: C 20 h 28 o 6 ; 1 HNMR (300MHz, CDCl 3 ) 6.24(d, J=2.6Hz, H-5'), 6.21(d, J=2.6Hz, H-3'), 5.18(m, H-3), 3.26, 2.51(m, H-10), 2.28, 2.26 (both s, 2×COCH 3 ) 1.40~2.49 (H-4~9), 1.37 (d, J=7.0Hz, 3-Me).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com