Preparation and application of 3-substituted-N-hexyl phenothiazine derivative
A phenothiazine derivative, phenothiazine technology, applied in electrical components, electrical solid devices, circuits, etc., to achieve the effect of good electroluminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
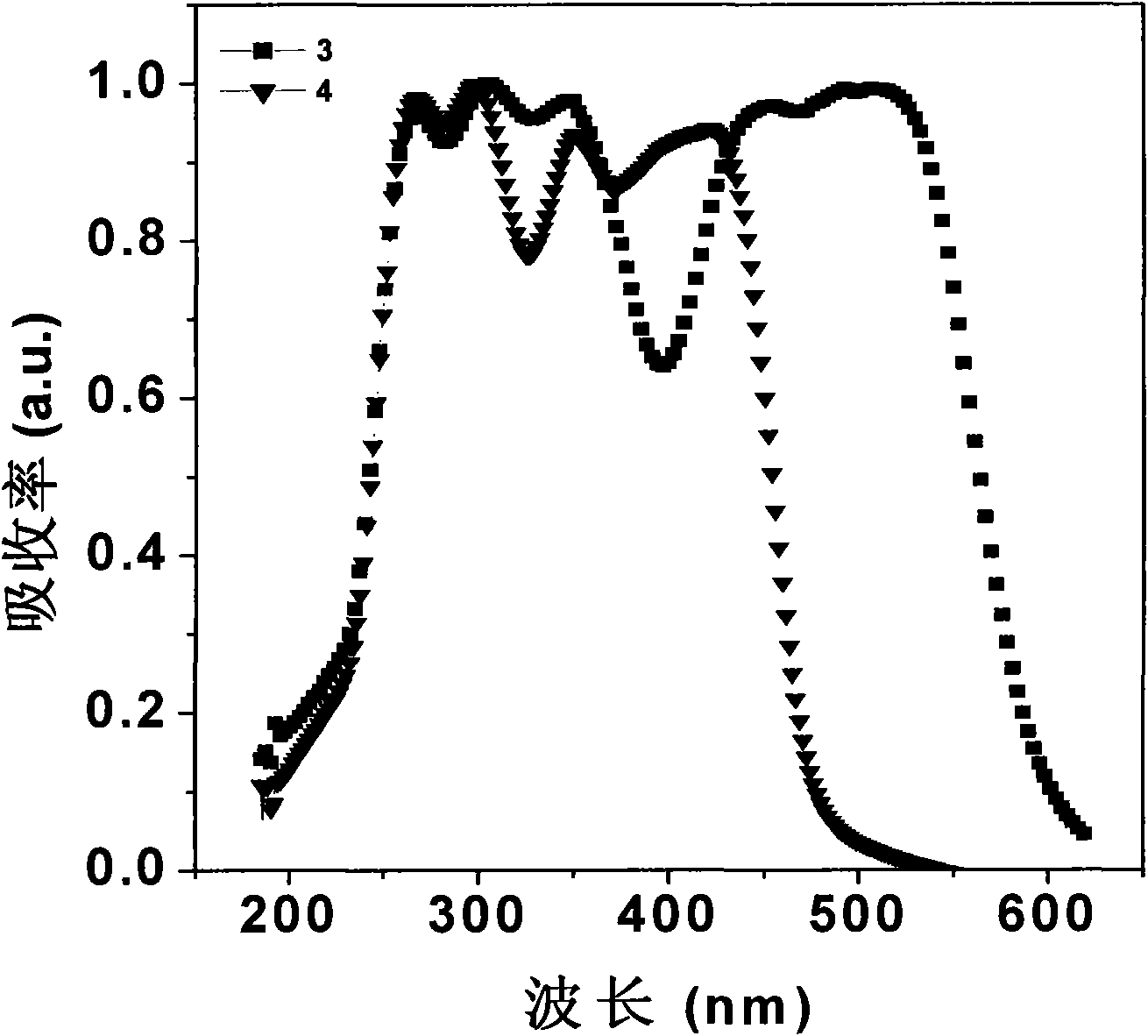
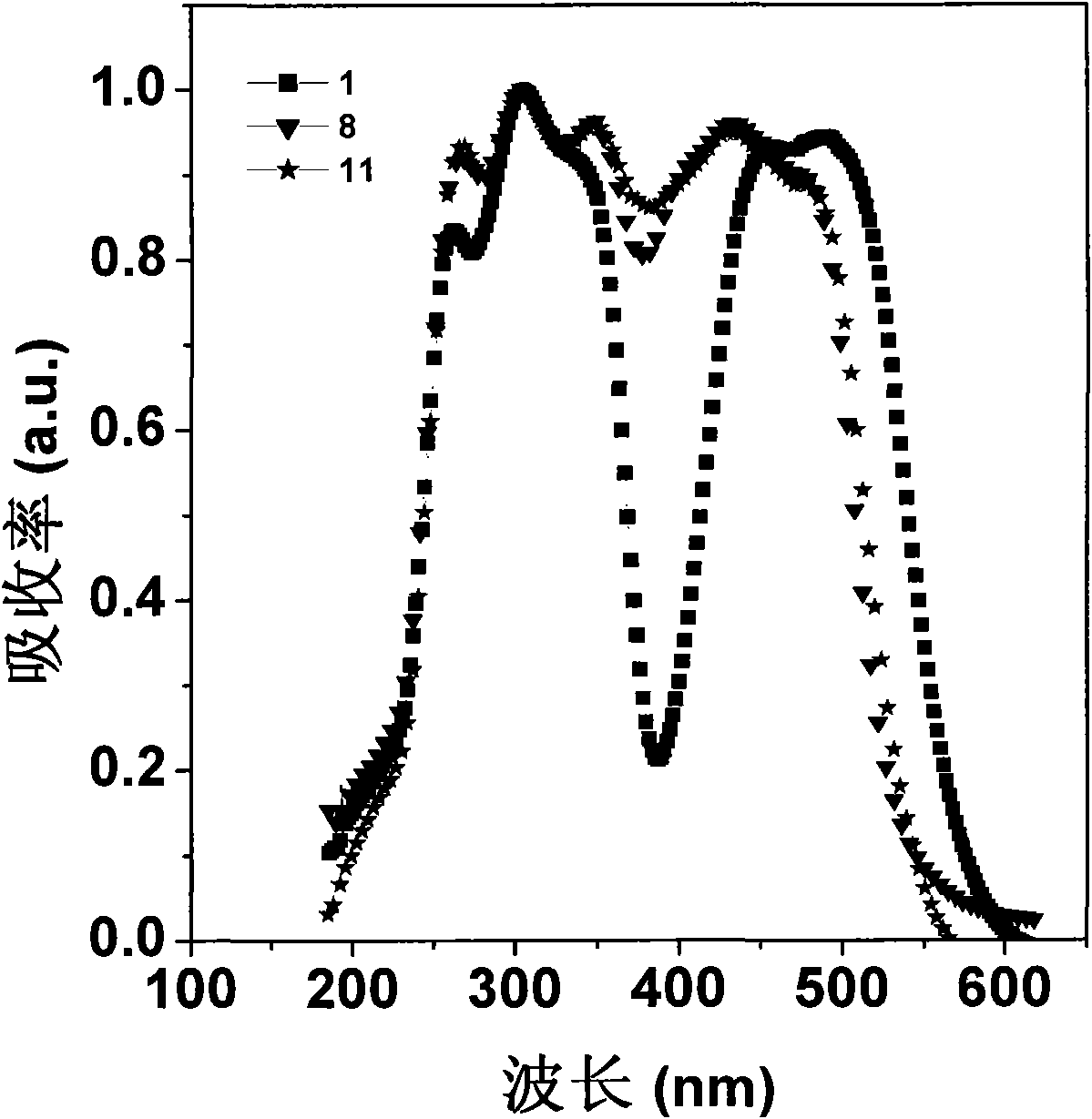
Image
Examples
Embodiment 1
[0071] Compound 1 Preparation:
[0072]
[0073] 3-Formyl-N-hexylphenothiazine (0.33 g, 1.1 mmol) and malononitrile (80.1 mg, 1.2 mmol) were added to a round bottom flask, and 15 mL of pyridine was added, and refluxed for 1 hour. The reaction mixture was cooled and poured into water, extracted with chloroform, washed with brine, dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain a reddish-brown crude product; the crude product was purified with a 300-400 mesh silica gel column, eluent: petroleum ether : acetone=15:1 (volume ratio), the red product-compound 1 (0.34 g, yield 87%) was obtained.
[0074] Compound 1:
[0075] Elemental analysis: measured value C% 73.31 H% 5.70 N% 12.03 S% 8.96;
[0076] Calculated value C% 73.54 H% 5.85 N% 11.70 S% 8.91
[0077] IR (cm -1 ) 2217 (CN), 960 (C=C).
[0078] 1 HNMR (CDCl 3 , 400MHz) δ: 0.88(t, 3H, CH 3 ), 1.32 (d, 4H, CH 2 ), 1.43(t, 2H, CH 2 ), 1.80(t,...
Embodiment 2
[0081] Compound 2 Preparation:
[0082]
[0083] Add 3-formyl-N-hexylphenothiazine (0.87g, 2.8mmol) in a 50mL round-bottomed flask equipped with a reflux condenser, and add n-butanol (27mL) and 3 drops of catalytic amount of piperidine, heat up Reflux, 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine-2-methanesulfonic acid (0.30g, 1.3mmol) was added to the reaction flask in four times, and the addition was completed. After reacting for 72 hours, n-butanol was distilled off under reduced pressure, purified by 300-400 mesh silica gel chromatography column, eluent: petroleum ether: ethyl acetate = 15:1 (volume ratio), and the first color band was collected to obtain purple Black product - compound 2 (190.3 mg, 19% yield).
[0084] Compound 2
[0085] Elemental analysis: measured value C% 67.87 H% 5.72 N% 10.39 S% 12.21;
[0086] Calculated C% 67.95 H% 5.95 N% 10.34 S% 11.83.
[0087] IR (cm -1 ) 1660, 937 (C=C), 1335, 1165 (SO 2 )
[0088] 1 HNMR (CDCl 3 , 400MH...
Embodiment 3
[0091] Compound 3 preparation:
[0092]
[0093] Add 3-formyl-N-hexyl phenothiazine (0.87g, 2.8mmol) in a 50mL round-bottomed flask equipped with a reflux condenser, and add n-butanol (27mL) and 4 drops of catalytic amount of piperidine, heat up Reflux, 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine-2-methanesulfonic acid (0.30g, 1.3mmol) was added to the reaction flask in four times, and the addition was completed. After reacting for 72 hours, n-butanol was distilled off under reduced pressure, purified by using a 300-400 mesh silica gel chromatography column, eluent: petroleum ether: ethyl acetate=15:1 (volume ratio), and the second color band was collected to obtain orange Yellow solid product-compound 3 (277.0 mg, yield 42%).
[0094] Compound 3
[0095] Elemental analysis: measured value C% 62.45 H% 5.67 N% 13.50 S% 12.16;
[0096] Calculated C% 62.40 H% 5.62 N% 13.48 S% 12.34.
[0097] IR (cm -1 ) 936 (C=C), 1333, 1163 (SO 2 ).
[0098] 1 HNMR (CDCl 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com