Atap peptides, nucleic acids encoding the same and associated methods of use
A nucleic acid and polynucleotide technology, applied in apoptosis-related proteins, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve problems such as unpleasantness and high toxicity in cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0165] The production of monoclonal antibodies is well known in the art; see, eg, Harlow et al., Antibodies: A Laboratory Manual, p. 726 (Cold Spring Harbor Pub. 1988). Monoclonal antibodies can be obtained by injecting a mouse or rabbit with a composition comprising the antigen and confirming the presence of the antibody product by removing a serum sample, removing the spleen to obtain B lymphocytes, and fusing the lymphocytes with myeloma cells To generate hybridomas, clone hybridomas, select positive clones that produce antibodies to antigens, and isolate antibodies from hybridoma cultures. Monoclonal antibodies can be isolated and purified from hybridoma cultures by techniques well known in the art.
[0166] In other embodiments, antibodies can be produced recombinantly, eg, by phage display or by combinatorial methods. Phage display and combinatorial methods can be used to isolate recombinant antibodies that bind to ATAP, ATAP-binding proteins, and / or ATAP receptor prote...
Embodiment
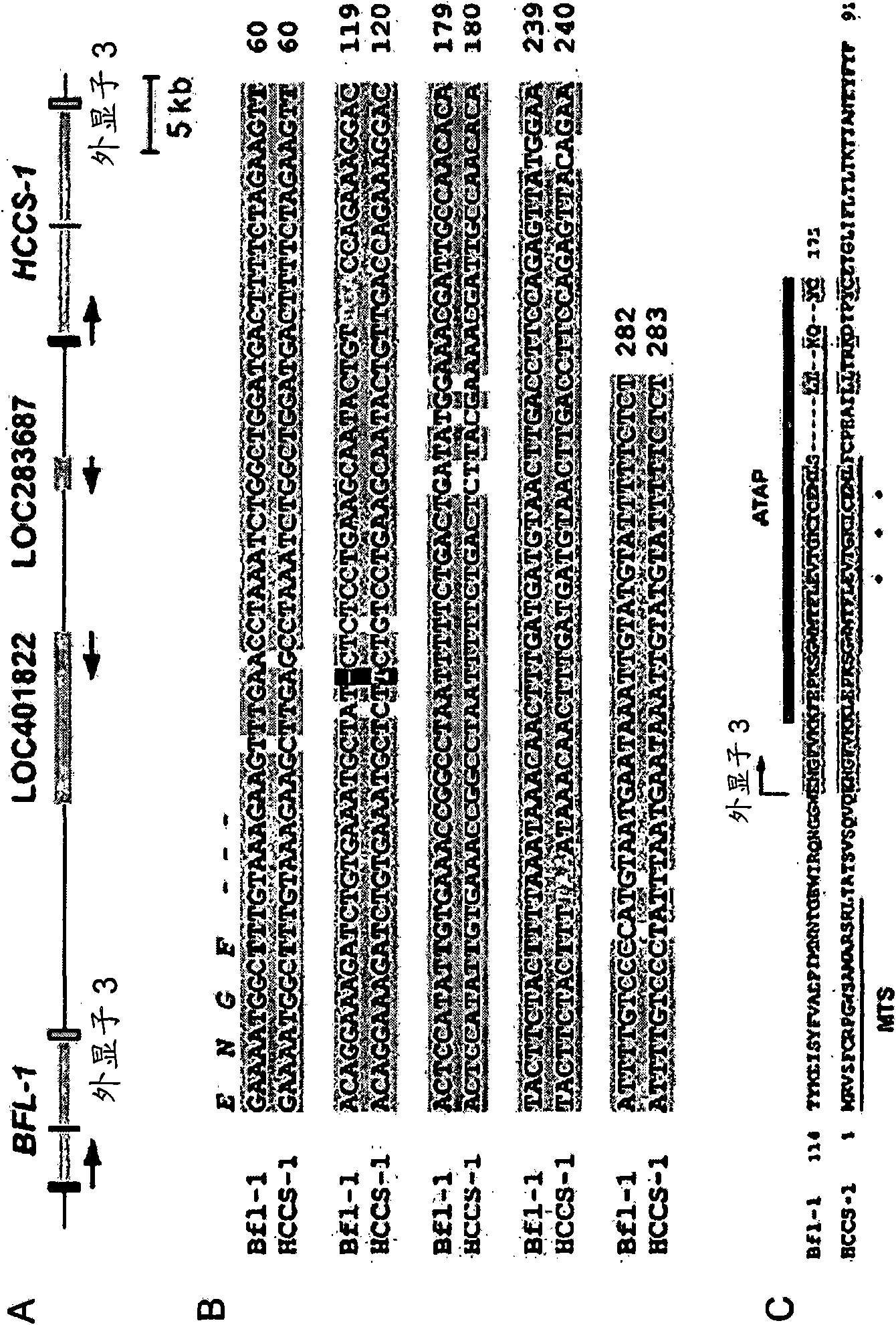
[0212] The amphipathic tail anchor domain is conserved in Bfl1 and HCCS1. The gene encoding Bfl1 (BCL2A1 or BCL2-related protein A1) is located on chromosome 15q24.3 and contains three exons, which are transcribed into two alternatively spliced variants, Bfl1(175a.a.) and Bfl1s(163a .a.) (Ko et al., 2003b). The tail anchor (TA) domain of Bfl1 (a.a.147-175) is encoded by exon 3. A database search revealed that exon 3 of BCL2A1 is conserved in HCCS1 (16.4 kb) located on 15q25.1 ( figure 1A). Sequence comparison of the genome sequences of BCL2A1 and HCCS1 revealed that the 8.8 kb fragment of BCL2A1 including exon 3 and the surrounding noncoding regions are highly conserved in HCCS1, which may be due to the duplication of 12 conserved gene segments .
[0213] The genomic sequence of HCCS1 also contains three exons, which encode 91 amino acids ( figure 1 A). The DNA sequence of exon 3 of HCCS1 is 95% identical to that of BCL2A1, and starts at E28 of HCCS1 and E141 of Bfl1 r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com