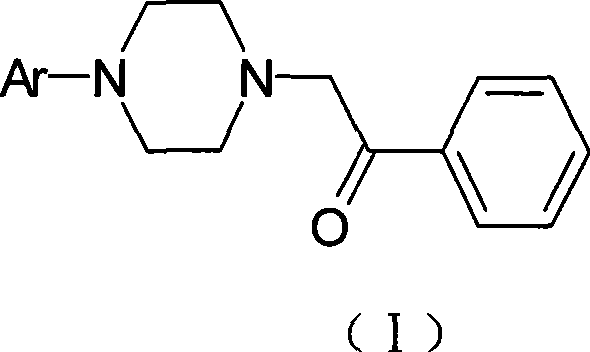
Use of arylpiperazine derivatives in preparing medicament for treating ache
A technology of use and medicine, which is applied in the field of use of arylpiperazine derivatives in the preparation of medicines for treating pain, and can solve problems such as renal dysfunction, easy addiction, respiratory depression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation of 2-[4-(2-methoxyphenyl)-1-piperazinyl]-1-phenylethanone
[0064] 4-(2-methoxyphenyl)-1-piperazine hydrochloride (46.7g, 0.20mol), α-chloroacetophenone (37.2g, 0.24mol), ethanol (480ml), sodium carbonate (50.4g, 0.5mol), was added into a 1000ml four-neck flask, and refluxed for 4 hours. TLC was followed until the reaction was complete. Cool and filter. The filter cake was dissolved in dichloromethane, the inorganic salts were filtered off, the filtrate was evaporated to dryness, recrystallized from ethanol, and vacuum-dried at 50°C to obtain 52.5 g of off-white solid with a yield of 84.7%. See Table 1 for the spectrogram data to confirm the structure.
Embodiment 2
[0066] Preparation of 2-[4-(3-methoxyphenyl)-1-piperazinyl]-1-phenylethanone
[0067] 4-(3-methoxyphenyl)-1-piperazine hydrochloride (46.7g, 0.20mol), the feeding amount and operation of α-chloroacetophenone, ethanol, sodium carbonate are the same as in Example 1, to obtain The off-white solid was 50.5 g, and the yield was 84%. See Table 1 for the spectrogram data for confirming the structure.
Embodiment 3
[0069] Preparation of 2-[4-(4-methoxyphenyl)-1-piperazinyl]-1-phenylethanone
[0070] 4-(4-methoxyphenyl)-1-piperazine hydrochloride (46.7g, 0.20mol), the feeding amount and operation of α-chloroacetophenone, ethanol, sodium carbonate are the same as in Example 1, to obtain The off-white solid was 53.5 g, and the yield was 85%. See Table 1 for the spectrogram data for confirming the structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com