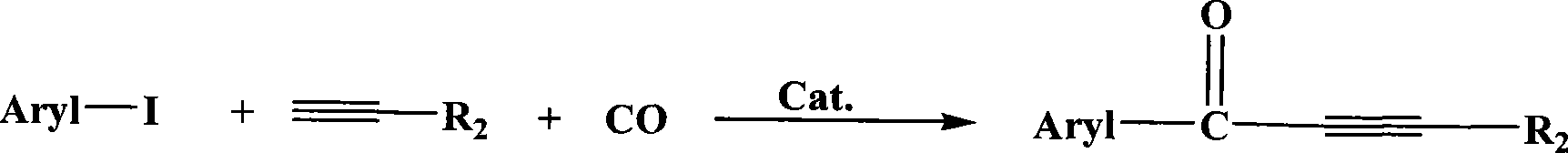Method for synthesizing alpha-beta unsaturated acetyenic ketone compounds by carbonylation
A chemical synthesis and unsaturated technology, applied in the field of α-β unsaturated acetylenic ketone compounds, can solve the problems of phosphine compound toxicity, homogeneous catalyst product and catalyst difficult to separate, and achieve high reactivity, low cost and good selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 5%Pd / C: 10mg, Et 3 N: 7.2mmol, iodobenzene: 2.5mmol, phenylacetylene: 3mmol, 4ml toluene, carbon monoxide pressure 2.0MPa, reaction at 130°C for 4h to obtain 1,3-Diphenyl-2-propynone. The conversion rate of iodobenzene is 96%, and the selectivity of 1,3-Diphenyl-2-propynone is greater than 99%.
Embodiment 2
[0025] Same as Example 1, without adding alkali, no 1,3-Diphenyl-2-propynone was obtained.
Embodiment 3
[0027] Same as Example 1, the base used is pyridine (7.2 mmol) to obtain 1,3-Diphenyl-2-propynone. The conversion rate of iodobenzene is 28%, and the selectivity of 1,3-Diphenyl-2-propynone is greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com