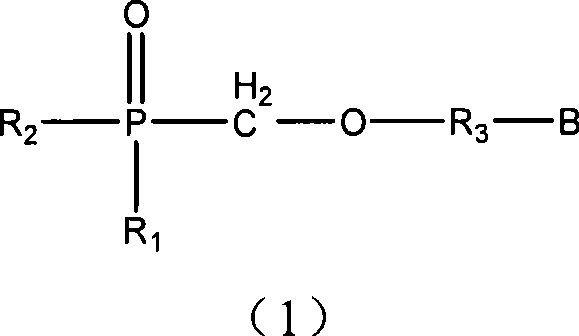
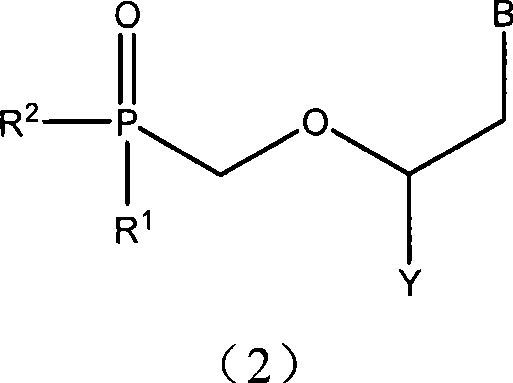
Nucleotide analogs and use thereof, and medicament composition containing nucleotide analogs
A compound and medicinal salt technology, which is applied in the field of pharmaceutical compositions containing such compounds, can solve problems such as DNA chain interruption, achieve the effects of reducing lattice energy, increasing bioavailability, and improving fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Example 1: 9-[2-[bis(trifluoroethoxymethoxy)phosphorylmethoxy]ethyl]purine (6)
[0119] In a 100ml single-necked bottle, add 1g of PMEA, add 50ml of thionyl chloride, add a catalytic amount of DMF, heat and reflux for 3 hours to obtain a dark red liquid, evaporate the thionyl chloride, add 30ml of anhydrous acetonitrile, stir to dissolve, and obtain a solution A; Dissolve 1.46g of trifluoroethanol and 0.6ml of anhydrous pyridine in 20ml of anhydrous acetonitrile to obtain solution B; add solution A dropwise to solution B at 70°C, after the addition, heat to reflux for 8h, concentrate, and silica gel Column chromatography, eluting with dichloromethane:methanol=30:1, yielded 0.20g of the product, yield 11.8%, melting point: 141.4-141.8°C,
[0120] 1 H-NMR (DMSO-d 6 )δ (ppm) 3.88-3.92 (m, 2H, 2-H), 4.09-4.12 (d, 2H, OCH2P), 4.31-4.34 (m, 2H, 1-H), 4.53-4.66 (m, 4H, OCH2CF3), 7.39(s, 1H, 2-H), 8.12(s, 1H, 8-H)
Embodiment 2
[0121] Example 2: 6-Ethoxycarboxamido-9-[2-[bis(trifluoroethoxymethoxy)phosphorylmethoxy]ethyl]purine (7a)
[0122] In a 100ml three-necked round-bottomed flask, add 1.75g 9-[2-[bis(trifluoroethoxymethoxy)phosphorylmethoxy]ethyl]purine, add 50ml of dry dichloromethane to dissolve, cool with ice, Add 0.32ml of anhydrous pyridine, dropwise add 0.35ml of ethyl chloroformate, react for 2h, add ice water, separate layers, separate the organic phase, CaCI 2 Drying, concentration, silica gel column chromatography, eluting with dichloromethane:methanol=30:1, the product was 1.74g, the yield was 85.6%, m.p: 122.7-123.2°C,
[0123] 1 H-NMR (CDCI 3 )δ (ppm): 1.32-1.37 (m, 3H, N6-CH3), 3.92-3.94 (d, 2H, OCH2P), 4.01-4.05 (t, 2H, N6-CH2), 4.29-4.38 (m, 6H , 2-H, OCH2CF3), 4.48-4.51 (m, 2H, 1-H2), 7.99 (s, 1H, 2-H), 8.712 (s, 1H, 8-H)
[0124] The following compounds were prepared in a similar manner:
[0125] 6-n-propoxycarboxamido-9-[2-[bis(trifluoroethoxymethoxy)phosphorylmethoxy]...
Embodiment 3
[0143] Example 3: n-propoxycarboxamide-5-fluoro-9-[bis(2,2,2-trifluoroethyl)-phosphonomethoxy]ethylcytosine (9a)
[0144] In a 100ml three-neck round bottom flask, add 0.86g of 5-fluoro-9-[bis(2,2,2-trifluoroethyl)-phosphonomethoxy]ethylcytosine and dissolve it in 50ml of anhydrous dichloromethane In the middle, cool on ice, add 0.2ml of anhydrous pyridine, dropwise add 0.2ml of isobutyl chloroformate, and stir for 2h. After the reaction was complete, the solvent was evaporated, followed by silica gel column chromatography, eluting with dichloromethane:methanol=30:1 to obtain 1.0 g of the product, with a yield of 72.7%, and a melting point of 84-85°C.
[0145] 1 H-NMR (CDCI 3 )δ (ppm): 0.97-0.99 (m, 3H, N6-CH3), 1.71-1.75 (t, 2H, N6-CH2CH3), 3.86-3.88 (m, 2H, OCH2P), 3.95-3.96 (m, 4H , NCH2CH2O), 4.13-4.15 (m, 2H, N6-OCH2), 4.38-4.45 (m, 4H, OCH2CF3), 7.42-7.43 (d, 1H, 4-H)
[0146] The following compounds were prepared in a similar manner:
[0147] 6-isobutoxyamide-5-flu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap